A practical approach to microbial testing to support non-sterile product stability
Posted: 3 December 2008 | Linda Skowronsky, Senior Development Microbiologist, GlaxoSmithKline | 1 comment
During stability, product testing is performed to ensure the product will continue to meet specified criteria of quality and strength through its expiration or shelf-life at the temperature and humidity required by specific markets. This article will not address the other stability requirement of continued efficacy during consumer use which is done by “in-use testing”, an important subject for another article.
During stability, product testing is performed to ensure the product will continue to meet specified criteria of quality and strength through its expiration or shelf-life at the temperature and humidity required by specific markets. This article will not address the other stability requirement of continued efficacy during consumer use which is done by “in-use testing”, an important subject for another article.
During stability, product testing is performed to ensure the product will continue to meet specified criteria of quality and strength through its expiration or shelf-life at the temperature and humidity required by specific markets. This article will not address the other stability requirement of continued efficacy during consumer use which is done by “in-use testing”, an important subject for another article.
Since Microbial Limit Testing (MLT), to include Microbial Enumeration (TAMC and TYMC) and Test for Specified Organisms; and Antimicrobial Effectiveness Testing (AET), if appropriate, are attributes that define the quality of a product, these tests should be considered and included as part of the stability program for both setting expiratio, and supporting on-going commercial stability. The required testing for microbiology is a bit ambiguous as written in ICH 6QA1 which, thankfully, leaves room to define testing conditions that make sense for the product under study.
There have been varied opinions regarding the amount and type of testing that is necessary to support the stability of the microbial attributes and efficacy of the many antimicrobial preservatives. Many programs have instituted testing at all the intervals and temperatures required for analytical analysis. In some cases for global markets, this has included tests for MLT and/or PET at 0, 1, 3, 6, 9, 12, 24, 36 months at 25°C /60%RH, 30°C/60%RH, 30°C/%65RH, 30°C/75%RH; with additional accelerated conditions of 40°C/75%RH. All this testing can amount to a lot of expensive, time consuming work when the answer to microbial stability question can be answered much more simply.
Once a good quality product has been developed using quality raw materials and good manufacturing practices, maintaining this quality on stability is a matter of controlling the factors that favour microbial growth which may be quite different than those necessary for survival. Temperature and humidity are extrinsic factors that control microbial growth and the intrinsic factors controlling growth in most formulations include:
- Low water activity
- Extreme pH levels
- Presence of antimicrobial compounds
Change in any one of these intrinsic factors due to alterations in extrinsic factors could change a static or hostile environment to a growth supportive environment2. In order to demonstrate that the environment was supportive, however, organisms would first have to be present and viable. A test showing “absence of growth” during stability storage would not necessarily rule out growth potential. Growth potential would be better determined using the AET where organisms are actually added to the product, or by water activity (Aw) measurements in non-aqueous formulas, or a combination of both tests.
This article will discuss rationale for microbial stability testing, ways to optimise the microbial limit and antimicrobial effectiveness testing, and criteria in the stability testing program in order to reduce testing levels and frequency. This approach requires getting into the microbe’s world to better see what they would prefer.
Microbial limit expectations
Test criteria
The purpose of the testing on stability is not only to ensure the product continues to meet the release limit, as is the general thinking; but also to ensure growth will not occur. If a material was found to have a count of <10 cfu/g at the initiation of the stability, and after six months was found to have 950 cfu/g, this would still meet a limit of 1000 cfu/g; yet there was an increase of >/= 1 log10. If we are to say “No Growth”, a limit defining growth would be needed otherwise arbitrary fluctuations in counts might be viewed as growth. Since organisms grow logarithmically, a better measuring stick for stability of microbial levels might be “</= 0.5 log increase from initial time point”. This measurement is described in USP<51> Antimicrobial Preservative Effectiveness where it defines “No Increase” as not more than 0.5 log10 increase from a previous measurement.
The next question is; why would one test for specified organisms on stability? If at release the product was Escherichia coli “absent” meeting the requirements of the test, would the discovery of E. coli at 12 months mean the formula was not stable? If the Total Aerobic Microbial Count (TAMC) showed an increase in counts then this may be true, but if not, the real issue was with the release and not the stability.
Temperature selection
One of the most important extrinsic factors influencing survival and growth is temperature. Most mesophiles, the organisms targeted using compendial test methodology, grow at 25°C-40°C. In selecting the optimum temperature that would likely support microbial growth in the stability program, it should be understood that microbial survival and growth differ in an optimal environment versus that in a sub-optimal environment. Under ideal conditions in culture, growth increases with temperature within the optimal temperature range of an organism. However, in the presence of antimicrobial factors, or in sub-optimal environments, the opposite is true; antimicrobials are more active and organisms are more sensitive to antimicrobials at higher temperatures. Beuchat reported that at any given Aw, an increase in temperature generally decreases the viability of fungal spores, while lower temperatures (above freezing) favour longevity3. Based on this information, it would make sense to perform Microbial Limits at the temperature most likely to favour growth (25°C-30°C); accelerated conditions of 40oC would not be of value since this temperature would be least likely to support growth and could actually be fatal4.
Test humidity selection for MLT
Now what about humidity? It is a common assumption to think that the higher humidity would be the more optimum for survival and growth because it would be expected that increased moisture would favour growth. However, it has been reported that survival is best in non-aqueous formulas at lower humidity (23%-46%RH); and survival actually decreases as the humidity increases until 95%RH is reached, after which there is sufficient moisture available for growth5. Organisms can survive better in dry environments than in sub-optimal moist environments due to the defense mechanisms which bacteria use as water activity decreases6. At extreme humidity >95% typically most solids have more problems beyond that of microbial growth, such as softening of tablets and caking of powders. Based on this information, the lowest RH would be preferred as this would favour survival. Since aqueous formulas have sufficient moisture present, humidity should have no affect on the growth potential in these formulas.
Test intervals for MLT
Now that we have selected the optimum temperature and humidity to test, what test intervals would be optimum? It has been reported that the lag phase is usually longer as the water activity decreases7. For this reason, a selection of an early interval such as one month might be warranted on a highly aqueous formula and longer periods of time are adequate in dryer materials.
If there are slow growing organisms present that are picked up later in time, this may be a function of the testing at release, rather than a formula stability issue. This can be illustrated in recalls that have shown presence of high levels of Burkholderia cepacia in highly aqueous formulas that were negative at release. Organisms that have developed a resistant form in sub-lethal levels of dilute product due to inadequate cleaning can grow quite well even in adequately preserved formula that would otherwise be stable. This was observed by this author when a product containing 10% Benzyl Peroxide gel with parabens and Germall II supported the growth of Burkholderia cepacia on storage. This organism was incapable of surviving when removed to a supportive environment and put back into product. Once the cleaning issue was isolated and resolved, this organism was no longer a problem.
Experience and knowledge of history of performance on similar formulas may justify use of minimising testing intervals. If you know the product is inherently hostile due to pH, water activity or preservative; and there is a low bioburdon at release, repeated testing will unlikely show any microbial changes.
Using water activity vs. MLT
In cases where there is insufficient moisture, such as in most solid dosages and ointments, water activity (Aw) is the more preferred measurement to ensure control of growth on stability since Aw is a characteristic of the formula unlike the microbial population. Use of this measurement for microbial stability is described in USP<1112> Application of Water Activity Measurement in Non-sterile Pharmaceutical Products. During development it is helpful to expose a solid dosage for several weeks outside its package to high temperature and humidity to develop a water activity profile. If the water activity doesn’t exceed 0.75 Aw, performing microbial limits on stability would be meaningless. If the water activity exceeds 0.75 Aw but the package can maintain the Aw below this limit as measured during stability, again, MLT would be meaningless8,9,10. In Figure 1 below, the relationship of water activity to survival is demonstrated. In this study, 0.1 ml or 0.01 ml volumes of diluent containing 106 A. niger spores, is inoculated onto tablets made with either Dicalcium Carbonate Dihydrate or Avicel. As can be seen in the chart, survival is greater on the tablets with the increased water activity.
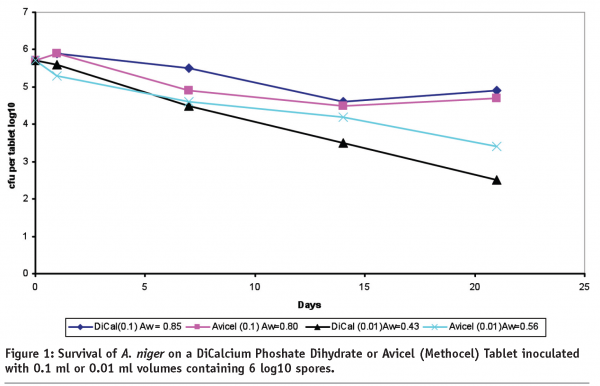

Mould growth in tablets is rare and elevated levels are usually found using poor quality raw materials, delayed or inadequate drying of blends or insufficient cleaning practices. There have been reports of mould and yeast growth on tablets stored in large containers in tropical regions with excessive heat and humidity11. Under these extreme conditions, control of moisture using desiccants or a blister with better moisture barrier would be necessary. If water activity cannot be controlled below the growth supportive limit, a preservative may be necessary. Adding a preservative should be avoided if possible, however, as justification to the regulatory authorities is often difficult it could delay the submission process.
Antimicrobial preservative effectiveness
Since the higher temperatures can degrade preservatives and potentiate other chemical activities, testing AET at the higher temperatures would be evaluating the worst case scenario. Accelerated testing is typically done during development, and with sufficient information, AET testing of commercial annual stability batches may not be necessary. During development, it is helpful to perform AET on a “base” formula minus preservative, flavours, fragrance or growth-limiting factors at their lowest levels. The pH should ideally be the expected range closest to that favouring growth. Use of organisms beyond that required by the compendia is also suggested during this testing phase. Knowledge of the “base” formula susceptibility aids in determining the level and type of preservative needed. Once the lowest level needed to control growth is determined, this level will be the lowest specified limit of preservative content. The complete formula can now be tested in the stability program with confidence that as long as the level is maintained above the minimum limit, growth will be controlled. This supporting information will provide a rationale to support reduced testing during stability to establish shelf-life; and may be eliminated from commercial stability provided the preservative level is monitored chemically.


Summary
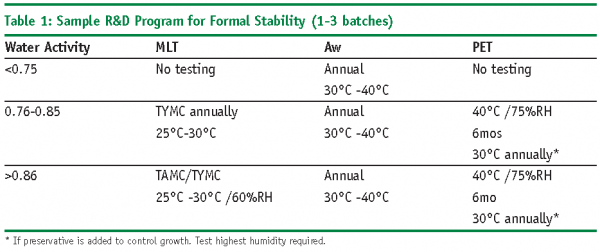
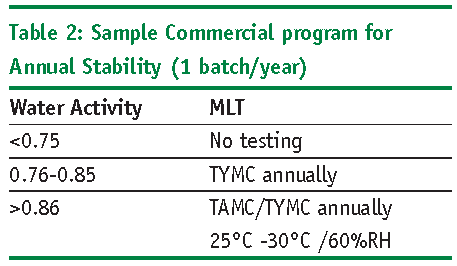
The following points should be considered when determining microbial testing on stability (Refer to Tables 1 and 2 for sample microbiology testing programs):
- Passing MLT during stability does not ensure microbial stability of the product
- Failing MLT during stability does not necessarily mean the product is not stable
- Water activity is a better tool for determining growth potential in non-aqueous formulas than MLT
- MLT on stability need only include TAMC and TYMC. No need to test for Specified Organisms again
- Early development testing of Aw and AET can reduce or eliminate MLT of non-aqueous formulas and PET during stability
- Test MLT at lower temps (25-30oC)
- Test MLT at the lowest %RH
- Perform AET on samples that have been stored at higher temperatures.
“Portions of this article were previously published in American Pharmaceutical Review Vol. 11, Issue 6 September/October 2008. Permission granted for publishing by Russell Publishing, LLC.”
References
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), “Stability Testing of New Drug Substances and Products (ICH Q1A (R2)).” ICH, November 2003
- Lenovich, L. Survival and death of Microorganisms a influenced by Water Activity. Water Activity: Theory and Application to Foods ed by Rockland L.B. Beuchat, L. R. (1987) p119-133
- Beuchat, L. Influence of Water Activity on growth, Metabolic Activities and Survival of Yeasts and Molds., Journal of Food Protection Vol 46, No 2 P. 135-141 (Feb 1983)
- Denyer, S., Baird, R. Guide to Microbiological Control in the Pharmaceuticals and Medical Devices (1990) p280-285
- Bloomfield,S.;Baird, R.; Microbial Quality Assurance in the Pharmaceutical, Cosmetics, and Toiletries Industries, 108-113
- Banwart, Basic Food Microbiology (1989)Chapman & Hall p 101-113
- Troller, J. Adaptation and Growth of Microorganisms in Environments with reduced Water Activity Water Activity: Theory and Application to Foods ed by Rockland L.B. Beuchat, L. R. (1987)p101-106
- United States Pharmacopeia 31 <1112> Application of water Activity Determination to Nonsterile Pharmaceutical Products
- Moir, P, Microbial Limit Testing- Reduced/Replaced by Equilibrium Relative Humidity TOPRA Regulatory Rapporteur , Vol. 5, No. 4 April 2008 p. 14-16
- Martinez, Jose, Microbial Bioburden on Oral Solid Dosage Forms, Pharmaceutical Technology, Feb. 2002
- Obuekwe, C. O., Obuekwe, I. F., Rafiq, M.; Surface Microbial Contamination in Some Commonly Available Tablet Dosage Forms a Department of Biological Sciences, and bEM Unit, SAF, Faculty of Science, Kuwait University, Kuwait; cDepartment of Pharmaceutical Microbiology, Faculty of Pharmacy, University of Benin, Benin City, Nigeria









I want to be sure, it is not a recommendation to test tablets when the storage conditions are not favourable, but when not stable and could support growth of microorganism,then one should test the tablets