Wyeth forges ahead
Posted: 28 September 2006 | | No comments yet
Is the pharmaceutical manufacturing environment of today becoming more challenging? There is increasing pressure to control or reduce costs because of the limitations on healthcare budgets. Asset utilisation, operating efficiencies and cycle times in the pharmaceutical industry generally compare unfavourably with other high technology industries. Despite the resources invested in compliance, many companies continue to struggle to meet the regulatory requirements, as evidenced by warning letters and consent decrees.
Is the pharmaceutical manufacturing environment of today becoming more challenging? There is increasing pressure to control or reduce costs because of the limitations on healthcare budgets. Asset utilisation, operating efficiencies and cycle times in the pharmaceutical industry generally compare unfavourably with other high technology industries. Despite the resources invested in compliance, many companies continue to struggle to meet the regulatory requirements, as evidenced by warning letters and consent decrees.
Is the pharmaceutical manufacturing environment of today becoming more challenging? There is increasing pressure to control or reduce costs because of the limitations on healthcare budgets. Asset utilisation, operating efficiencies and cycle times in the pharmaceutical industry generally compare unfavourably with other high technology industries. Despite the resources invested in compliance, many companies continue to struggle to meet the regulatory requirements, as evidenced by warning letters and consent decrees.
The desired state
The recognition that the current manufacturing and compliance model is not sustainable has resulted in publication of a number of guidance documents seeking to provide a roadmap for change. The concepts described in the FDA PAT guidance, ICH Q8 Pharmaceutical Development, ICH Q9 Quality Risk Management and forthcoming ICH Q10 Pharmaceutical Quality Systems documents provide a framework for a new quality/regulatory paradigm and facilitate pharmaceutical manufacturing to progress to the ‘desired state’. This is where product quality and performance are ensured through the design of effective and efficient manufacturing processes and product and process specifications are based on a mechanistic understanding of how formulation and process factors affect product performance. This will enable continuous real time quality assurance, replacing the present reliance on finished product testing to confirm quality.
Wyeth PAT projects
To take advantage of the opportunities offered by the new quality paradigm, a few years ago Wyeth Pharmaceuticals created a PAT strategy (Table 1). This sought to enhance manufacturing efficiencies and foster a wide variety of initiatives at the various manufacturing sites for both commercial products and also development products. Other divisions of Wyeth have also been pursuing PAT initiatives with strategies tailored to their businesses.
Coating process monitoring using TDLAS
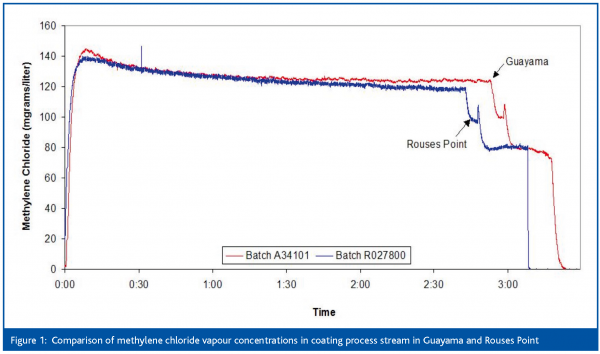
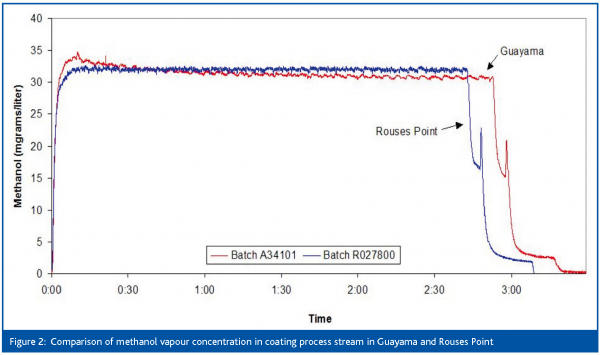
An early example of successful process monitoring was the application of Tunable Diode Laser Absorption Spectroscopy (TDLAS) to measure the solvent concentrations in the drying air during coating of particulates. The objective was to use TDLAS to measure methanol and methylene chloride concentrations during Glatt Wurster spray coating and correlate the concentrations to events during the coating process, other process parameters and selected final product quality attributes. TDLAS offers a number of advantages for this application: data can be acquired continuously in real time, with high sensitivity and specific to a solvent. Reliability is assured because the laser is solid state and uses a fiber optic link to the processor so there is no operator exposure during data collection. During the testing it was shown that the sensor data could be related to several operational events, including a rapid solvent increase during a coating failure. Process stream solvent vapour concentrations also correlated with the condenser temperature during normal spray coater operation. Figures 1 and 2 (previous page) and Table 2 show some of the data that was generated that demonstrated process equivalency during a technology transfer of the coating process from Rouses Point, NY to Guayama, Puerto Rico. This data was used to obtain a reduction in the regulatory filing requirements for the transfer: only one month of accelerated stability data was required and no bioequivalence study was necessary. This enabled the transfer project to be accelerated and savings in excess of $50 million to be achieved.
Reaction end-point monitoring by refractive index
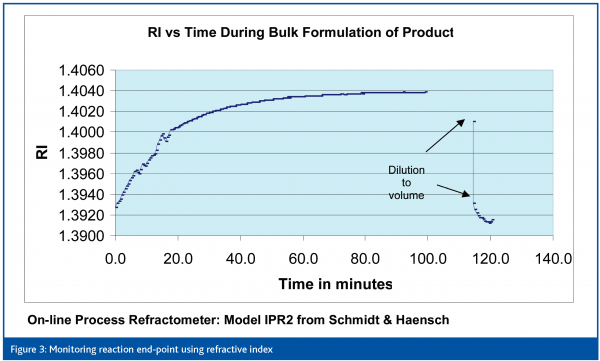
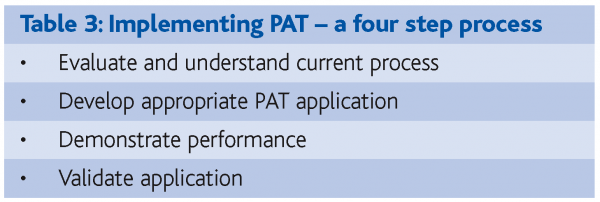
In another project the reaction end-point was monitored using an on-line refractive index technique. The reaction was the first stage of the manufacturing process for an IV formulation and comprised a two compound acid-base reaction where the end point was determined by at-line measurement of pH. Once the end point was reached filtration was used to remove excess solids. Refractive index measurement was selected to monitor the progress of the reaction because there is a linear relationship between refractive index and the concentration of dissolved solids that is not influenced by colour or turbidity. Refractive index sensor technology is mature and sensors are rugged and stable, enabling repeatable, accurate calibration when incorporated in the re-circulation line of the process vessel. A four-step implementation process was followed (Table 3) and the application was successfully validated. Figure 3 shows how the refractive index changes as the reaction proceeds, and it was confirmed that real-time reaction end-point detection was possible. However, despite this success Refractive Index monitoring has not yet supplanted the current, arguably inferior, pH test. Why is this? Probably the major reason was the failure to fully involve the Quality Assurance function in the planning and implementation of the project, so the objectives of the different parts of the organisation were not aligned.
PAT project learning
From these projects and others, a number of critical success factors have been identified for implementation of PAT. These include the need for multi-disciplinary project teams, a clearly defined implementation process and a strong business rationale. One of the goals of PAT is process understanding: ideally PAT measurements should focus on the product attributes, rather than, say, the equipment operating parameters. However, although not a product attribute, the solvent concentration data gathered from the TDLAS clearly contributed to process understanding and was used creatively to achieve a significant business benefit. Process monitoring appears to be relatively straightforward and helps develop process understanding, but moving to process control and continuous real time quality assurance presents more significant challenges. The Wyeth Pharmaceuticals strategy has been updated to reflect these lessons and other experiences.
Benefits of early process understanding
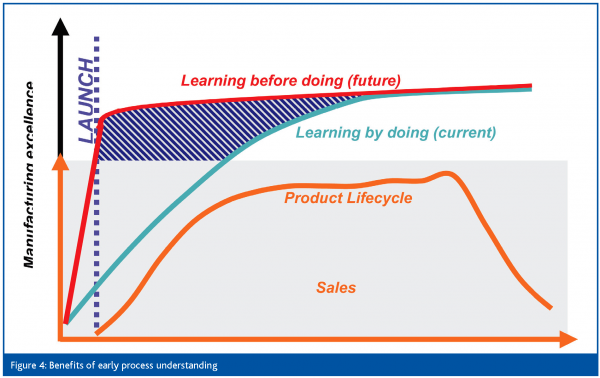
An analysis of new product launches showed that significant financial benefits could be obtained if greater process understanding could be developed earlier in the product life cycle to optimise manufacturing processes or eliminate defects (Figure 4). In one product it was estimated that cost of goods savings of $10 million and a capital expenditure avoidance of $50 million could have been achieved if a faster coating spray rate had been available from launch. In another product a minor defect caused production losses of $2.5 million and delays in production resulting in further losses.
New Pharmaceutical Development Centres
How can process understanding be achieved earlier in the product life cycle? Wyeth has recently started a project to construct two new Pharmaceutical Development Centers (PDCs) at Newbridge, Ireland and Guayama, Puerto Rico, with effectively identical design, technology and operation in both sites. These new laboratories are designed to optimise process robustness and development by integrating development and manufacturing in the commercial facility to support pipeline products and existing product portfolios.
Key design and operating principles include flexibility, scaleability, cleanability and containment, with the labs equipped to operate at 1-5kg and 5-50kg scales. Capital expenditure is expected to be in the region of $50-100 million over 3-4 years and significant annual savings are expected in product development costs when the labs are operational. Although new products are expected to be the priority for the PDCs, increasing the understanding of the manufacturing processes of existing products will also be important and, in addition, the Centres may be used to explore new technologies and to manufacture clinical supplies.
PDC charter
The key element of the charter for the Centres is to ensure process robustness so Quality by Design and PAT are at the heart of the design and operating principles. The focus will be to understand process variability, complete process range studies and determine the critical control parameters. This will improve the product and process characterisation to ensure the shortest possible development time, and mitigate the risk of launch delays and post-launch manufacturing problems. The Centres will play a critical role in the transfer of knowledge from R&D to the commercial manufacturing site – indeed the principle output of the PDCs is knowledge, not product.
What PAT capabilities do the Centres need? Design of Experiments and multi-variate statistical analytical tools will be needed to maximise the information gained from experiments. Clearly process monitoring using multi-variate analysers (e.g. NIR spectroscopy etc.) as well as ‘traditional’ univariate sensors (e.g. thermocouples) is a basic requirement, but how important is process control for the PDCs? Although some work will be required to implement control models in the commercial facility, the ability to develop and test process control models during development will help to verify and demonstrate process understanding and robustness, and accelerate progress towards continuous real-time quality assurance in the commercial product.
PAT data management system
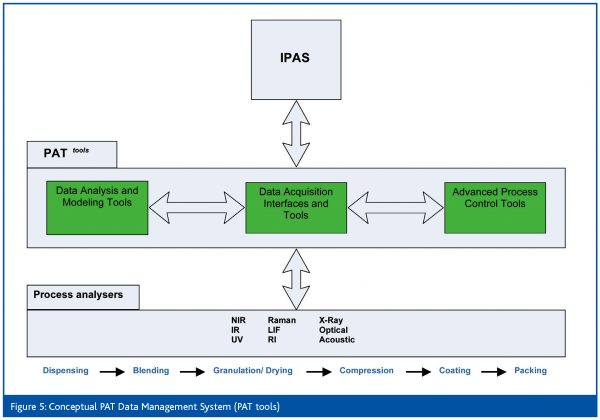
Because PAT applications are data intensive, establishing the appropriate PAT and data management architecture for the PDCs is essential. This management system – termed ‘PAT tools’ in the PDC project – must provide interfaces to all components of the PAT environment (instruments/analysers, systems, analysis tools, databases etc.) and manage the communication of status, events, data and meta-data between the components. It needs to ensure that timely measurements are available to PAT data analysis tools and outputs from those analyses can be input to the PAT process models to control the manufacturing process, via advanced process control techniques. It is worth noting that the system envisaged will use three of the four tools described in the FDA Guidance for PAT – multivariate data analysis, analysers and controls. The PDCs will also contribute to the use of the fourth tool, Continuous Improvement and Knowledge Management, but this must be a major feature of a product during routine commercial manufacture, unlike the present paradigm where manufacturing processes often remain largely unchanged during the product life cycle.
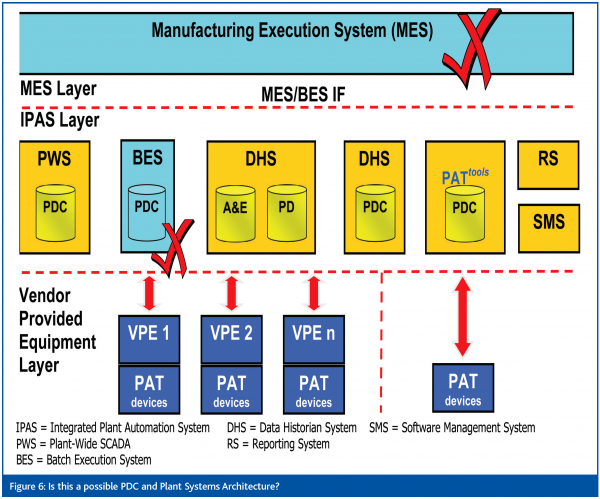
The PDC data management architecture must integrate with the plant automation systems (IPAS) and also provide storage of PAT data and process models – a conceptual model is shown in Figures 5. Certain practical problems must be overcome and decisions taken on the configuration of the ‘PAT tools’ system. For example (Figure 6), should the system be interfaced with the plant MES system? Can the Data Historian System handle univariate and multivariate data? Can some types of PAT analyser (e.g. NIR) be considered ‘generic’ i.e. potentially applicable to multiple products and built into the PDC equipment, or should analysers be purchased for, and dedicated to, specific products? The PDC project team is now grappling with these and other questions in the detailed design phase of the project, and is developing a User Requirements Specification for the PAT data management system.
Regulatory flexibility
In moving towards the desired state both companies and regulators need to understand how regulatory flexibility can be exercised when the concepts of the new quality paradigm are applied to pharmaceutical manufacturing. The EFPIA PAT Topic Group prepared a mock P2 submission document to enable discussion on the implementation of Quality by Design and PAT principles. This has been valuable in illustrating how Quality by Design and PAT concepts might be incorporated in regulatory submissions. The EMEA PAT Working Group recently published a Reflection Paper, ‘Chemical, pharmaceutical and biological information to be included in dossiers when Process Analytical Technology (PAT) is employed’, that provides guidance on European PAT regulatory submissions. Ultimately, real submissions are needed from the industry to help determine how knowledge should be transmitted to the regulators and enable them to develop guidances for assessors, inspectors and applicants. Wyeth has met with the EMEA PAT Working Group to discuss regulatory filings incorporating Quality by Design approaches. Wyeth has also met with the FDA PAT team to discuss various projects and is a participant in the FDA’s CMC Pilot Program. The ASTM International organisation is trying to develop consensus standards for PAT and Wyeth is a participant in the process to develop and agree these standards.
Accelerating towards the desired state
Wyeth has gained experience with a wide variety of PAT applications and is investing in the new Pharmaceutical Development Centres, where PAT and Quality by Design will be core operating principles. The regulatory submission strategies for filings incorporating these new concepts are being developed with knowledge gained from working with the regulatory authorities. Many challenges remain but the steps being taken will accelerate progress towards the ‘desired state’.
Acknowledgements
I would like to thank the following for contributing examples and information for the article: Doug Becker, Thomas Navedo, David Gibson, John Sourke, Martin Wallace (Wyeth) and John Townend (Jacobs Engineering).