Hoth Therapeutics’ topical therapy shines in cancer toxicity trial
Posted: 23 January 2026 | Catherine Eckford (European Pharmaceutical Review) | No comments yet
Achieves 100 percent response in phase II data highlighting HT-001’s potential as a supportive oncology treatment to address a common EGFR therapy burden.
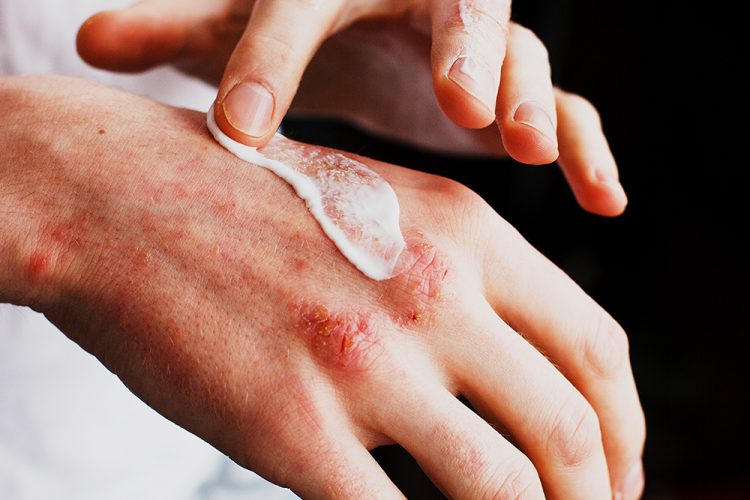

US biopharma company Hoth Therapeutics’ topical therapy HT-001 has shown strong promise as a new standard for care for cancer patients experiencing toxicity-related symptoms.
Patients on epidermal growth factor receptor inhibitors (EGFR) inhibitor therapies achieved a 100 percent response from HT-001 in interim phase II data from the open-label pharmacokinetic (PK) cohort of the ongoing phase II CLEER-001 clinical trial.
Seeing 100 percent clinical response in the open-label PK cohort… underscores the potential of HT-001 to improve the treatment experience for cancer patients receiving EGFR inhibitors”
Robb Knie, Chief Executive Officer of Hoth Therapeutics, said: “Seeing 100 percent clinical response in the open-label PK cohort, along with [approximately] 50 percent reduction in disease severity and additional improvements in oncology toxicity and patient-reported symptoms, underscores the potential of HT-001 to improve the treatment experience for cancer patients receiving EGFR inhibitors. We are encouraged by the breadth and consistency of these findings as the CLEER-001 trial continues.”
Furthermore, HT-001’s gel formulation enabled an improvement of approximately 34 percent in oncology toxicity and a reduction of around 37 percent in pruritus (patient-reported). Notably, all evaluable patients reached the low-severity disease range. Improvements were observed as early as Week 3 and were maintained through Week 6, demonstrating both rapid onset and durability of response.
HT-001 was well tolerated by patients in the open-label PK cohort. Hoth reported no unexpected safety signals.
Overall, the treatment could offer a potential advance in managing common toxicity burdens associated with treatment of EGFR inhibitors, drugs that have proved to be a key therapeutic tool in major cancers such lung and colorectal.
Promising interim phase II data released by Hoth last year demonstrated the potential of HT-001 in addressing EGFRi-associated skin toxicities resulting from cancer treatment.
Like the most recent data from Hoth, in this portion of the study, 100 percent of patients achieved the primary endpoint, as demonstrated by significant improvement in skin toxicity at six weeks.
Related topics
Clinical Development, Clinical Trials, Data Analysis, Drug Development, Drug Safety, Industry Insight, Research & Development (R&D), Therapeutics









