Safe as houses: the market impact of safety syringes in hospital and home drug administration
Posted: 6 December 2019 | George I’ons (Owen Mumford Pharmaceutical Services) | No comments yet
As the demand for safety-syringes increases, the market for pre-filled safety-engineered devices will increase. This article outlines the main drivers behind this growth.

Governments and healthcare systems are under growing pressure to remain effective despite staff shortages, lack of funding and overcrowding of their facilities. This is often a consequence of ageing populations affected with chronic diseases like diabetes, rheumatoid arthritis and psoriasis which typically require regular subcutaneous injection. One measure being taken to tackle these issues in the EU and USA is the drive towards self-administration of drugs for patients suffering from these conditions. Carers are also increasingly performing injections at home or relying on visiting nurses to deliver treatment.
While hospital settings are clearly regulated by the 6 November 2000 Needlestick Safety and Prevention Act1 in the US and by the EU Directive 2010/52/EU on the prevention of sharps injuries implemented in 2014 in Europe, this does not apply to home environments. In healthcare settings, needlestick injuries fell by around 30 percent in the US, subsequent to the Prevention Act, but even in hospital settings there is progress to be made before full compliance is achieved and half of non-hospital healthcare settings are in violation of OSHA law.2 With increasing numbers of patients self-administering treatment in their home each day or receiving treatment from carers and visiting nurses, the issue of needlestick safety during injection has spread far beyond the strictly clinical setting.
Self-administration of syringes
The US Bureau of Labor Statistics expects “personal care aide” and “home care aide” to be the second and third fastest growing job roles during 2012–2022, clearly indicating that there is a fast-growing portion of workers who need to be protected from needles within patient homes. Although home care aides typically do not use sharps themselves, there is a body of evidence showing that they frequently find themselves pressured to carry out administration and that sharps injuries in a home care setting are often caused by incorrect disposal of sharps.
With patients being encouraged to self-administer to manage their conditions, disposable delivery devices such as pre-filled safety syringes for subcutaneous delivery are becoming a key tool. However, the European Biosafety Network has found that sharps prevention practice compliance is as low as 60 percent, demonstrating the variety of issues likely to further drive this shift towards increased use of safety-devices.


Growth of the market
In addition to pressures on healthcare systems, there are several other factors driving self-administration and the market for safety-engineered pre-filled syringes specifically: population age, patient dexterity, the need to eliminate medical errors and a new influx of biosimilar drugs.
Populations in the Western world are ageing, but they are also presenting a higher number of people suffering from morbidities4 such as diabetes or rheumatoid arthritis, heart disease, Crohn’s disease, cancer and many others. Healthcare systems are finding it is not cost effective to treat this growing number of patients who often need daily treatment within a clinical setting. The dexterity, or lack thereof, of infirm or older patients is a major factor driving the market for pre-filled safety syringes as devices used by these patients need to be easy to use, require minimal force to activate and include passive safety mechanisms to reduce additional activation steps.
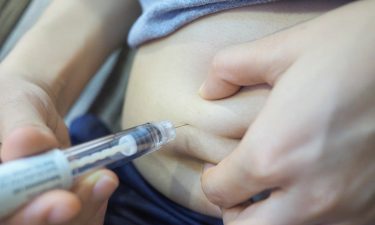

Another factor driving the increase in pre-filled devices in the US and Europe is an increase in pressure to eliminate medication errors further to several scandals5 reported in the national press. Pre-filled syringes could assist with ensuring that the correct drug is delivered accurately and in the appropriate dose, significantly reducing the risk of medical errors.
The dexterity, or lack thereof, of infirm or older patients is a major factor driving the market for pre-filled safety syringes”
Finally, reports6 clearly indicate that safety-engineered pre-filled syringe use is growing strongly, highlighting that pharmaceutical and biotechnology firms are committing to offering their users safety features for any administration setting. A recent report7 highlights some of the major drivers of the growing demand for safety-engineered devices as: improved regulatory compliance; higher standards of protection of all healthcare workers; protection of users from accidental injury and medical error; response to pressures on healthcare systems; the drive towards regular self-administration fuelled by new biological therapies and competitive biosimilar markets and increased demand of easy-to-use injectable therapies resulting from ageing populations. New combination products are therefore featuring safety elements which are thought to provide brand advantage.
Conclusion
Pre-filled safety-engineered device demand will therefore continue to grow as safety device demand increases across the globe, driven by various factors. As the market leading category, these devices respond to the range of user needs requiring protection from needle stick injuries (NSIs), dosage accuracy, high usability and safety mechanisms.
About the author
George I’ons has worked at Owen Mumford since 2006. His current work focus involves deciphering device requirements in the rapidly changing pharmaceutical and biotech sectors. In his previous roles he worked closely alongside R&D to develop devices for a variety of global pharmaceutical and diagnostic clients.
References
- Tatelbaum MF, Needlestick safety and prevention act, Pain Physician. 2001 Apr;4(2):193-5.
- Medical Devices:Evidence and Research, Vol 10, Clinical, economic, and humanistic burden of needlestick injuries in healthcare workers, 2 May 2017
- Market Data Forecast, Safety Syringes Market by Technology, Oct 2018
- See, for instance: The Lancet, Ageing: a 21st century public health challenge? July 2017; The Kings Fund, Making our health and care systems fit for an ageing population, Mar 2014
- See, for instance: The Telegraph, NHS drug errors may be causing up to 22,000 deaths every year, 23 Feb 2018
- Market Data Forecast, Safety Syringes Market by Technology, Oct 2018
- Owen Mumford Pharmaceutical Services, Safety First, September 2019
Related topics
Drug Delivery Systems, Drug Markets, Industry Insight, Vaccine Technology
Related organisations
Related diseases & conditions
Cancer, Crohn's disease (CD), Diabetes, heart disease, Psoriasis, Rheumatoid arthritis (RA)









