Pioneering a full-ecosystem microbiota approach to improve survival outcomes in life-threatening diseases
Posted: 16 December 2020 | Hervé Affagard (MaaT Pharma) | No comments yet
The human gut contains the largest ecosystem of microbial species having complex host-microbiome interactive networks that contribute to our physiology and regulate our immune system. An imbalance in this host-microbiome interaction is associated with disease and the ability to restore or stabilise the network by introducing a rich and diverse cocktail of microbial species can play a vital role in treating diseases. French biotech company, MaaT Pharma, has developed a differentiated approach to re-establish the full diversity of microbial species in the gut using its standardised, full-ecosystem microbiome restoration biotherapeutics and is expanding this approach with a breakthrough fermentation technology.

WE SHARE our bodies with trillions of symbiotic, commensal microorganisms – bacteria, fungi, viruses and other microbes. Collectively, they are called the human microbiota and all the genes they contain are called the human microbiome. The microbes that inhabit our various organs including the gut, skin and respiratory tract have a significant impact on how our bodies function in health and disease. Interactions between a host and their microbiome are complex, yet symbiotic, which are now being teased apart to understand their roles in human physiological functions. The complex communities of microbes that consist of thousands of distinct species vary enormously between each person and the microbiota in the gut have attracted a lot of interest over the last few years.
The gut microbiome
There are hundreds of distinct bacterial species in the gut comprising both pathogenic (‘bad’) and beneficial (‘good’) bacteria. The colonisation of our gut microbiota starts at birth, in co‑evolution with our immune system, and rapidly changes over time and throughout our lives based on the environment, diet and drugs we take such as antibiotics or intense treatments like chemotherapy. These commensal bacterial communities can regulate our immune system by presenting antigens and producing molecules that actively modulate its functions, providing our cells with nutrients and preventing pathogenic bacteria and viruses from colonising our gut. Research into this influence on our biology has yielded several insights for diseases like cancer, metabolic and CNS diseases and autoimmune disorders. An imbalance of the gut microbiota (or ‘dysbiosis’) has been correlated with multiple pathological states, suggesting their important role in the pathophysiology of the disease and the ability of their human host to respond to certain treatments.
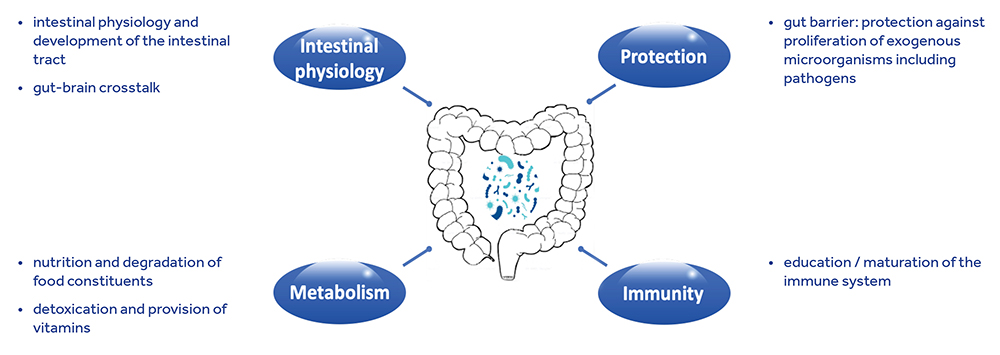

Figure 1: Impact of the gut microbiome on the host. ©Joel Doré
The microbial full-ecosystem concept
Like a vegetable garden, which functions as a result of many organisms working together in association above and below the ground, the complex community of microbes that inhabit the gut contributes to the whole function and there are myriad networks connecting them to each other and their host. Indeed, it has repeatedly been shown in the literature that the diversity (ie, number of different species) and richness of the gut microbiome correlates with health. Each species is part of a complex biodynamic network of chemically and physically interacting species, which can influence their individual behaviours and that of our body. Species co-operate (and sometimes compete) with each other for nutrients and also interact with host cells and, under healthy conditions, the resulting ecosystem achieves a balance between the host and the microbiota, or ‘homeostasis’. Richness and diversity are necessary to achieve appropriate metabolic and protective functions, as well as a positive influence on the immune system. After an applied stress, the ability of an altered ecosystem to restore itself to a functional state is dependent on microbial diversity and the effectiveness of each species to respond to external and internal changes. The higher the diversity and richness of species, the better their ability to restore, maintain or stabilise host‑microbiome balance and function.
The analytics platform allows the discovery of novel disease targets, the identification of biomarkers for microbiomerelated conditions and the rational design of drug candidates”
The first causative evidence of how the gut microbiota shapes our biology came from studying faecal microbiota transfer (FMT); this technique, consisting of administering the stool of healthy donors to receivers suffering from a pathological state, has been experimentally tested in multiple diseases such as diabetes, graft-versus-host disease or infections. For example, in Phase III clinical trials, FMT was shown to be successful at treating infections caused by the Clostridium difficile bacterium. This demonstrated that the gut ecosystem can be manipulated to prevent disease.
Advances in machine learning programmes that can be applied to the large datasets generated by multi-omics technologies such as metabolomics, proteomics, transcriptomics and metagenomics while studying the gut microbiota make it possible to effectively find correlations in the complex interactive network between the host and their microbiome. Extrapolating this information to healthy individuals as well as patients can help identify biomarkers and therapeutic targets for many diseases and, at the same time, help tailor the microbial consortium in the drug to particular diseases.
Patients undergoing repeated rounds of antibiotics and harsh treatments like chemotherapy have a severely reduced, or dysbiotic, microbiota in the gut in terms of both overall abundance and diversity, leading to an under-performing immune system. This favours the growth of pathogenic bacteria that invade the empty ecological niche, further challenging the already compromised immune system and reducing quality of life and survival chances for the patient. Our novel class of biotherapeutics aim to restore microbial imbalance and improve patients’ lives, as well as increase survival outcomes in life-threatening diseases. Our product candidates are characterised by both safety and a high richness in microbial species, thereby creating the most complete approach to restore the microbiome, boost the patients’ immune system and thus protect against inflammation and further infections.
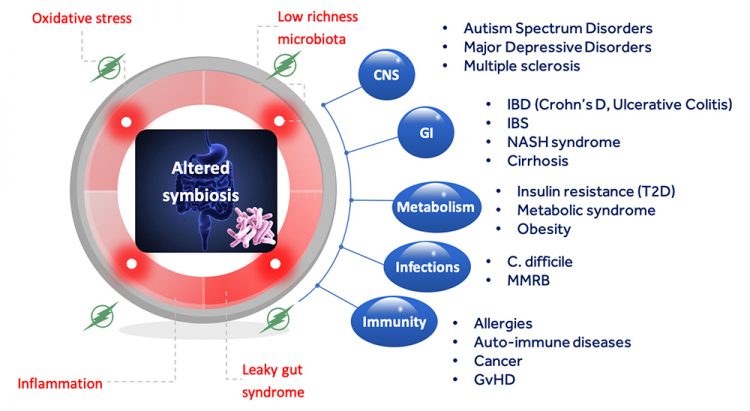

Figure 2: Diseases correlated with an imbalance in the host-microbiota symbiotic relationship. © Joël Doré
Current pipeline
Our proprietary microbiota computational data science platform, GutPrint®, forms the basis for our microbiome restoration biotherapeutics. The analytics platform allows the discovery of novel disease targets, the identification of biomarkers for microbiome-related conditions and the rational design of drug candidates. Our lead product candidate, MaaT013, is an enema formulation derived from pooling the full intestinal ecosystems of stringently-vetted healthy donors and is being investigated in a Phase II clinical trial in patients with steroid-refractory acute graft-versus-host disease (GvHD). GvHD is a severe complication that often occurs when cancer patients receive an allogeneic stem cell transplant. In GvHD, the donor’s immune cells start to target and react against the recipient’s organs, often leading to death. Current treatments mainly rely on corticosteroids that suppress the immune system; however, the mode of action of our products is to enable the body’s immune system to regain its balance. MaaT013 has been granted Orphan Drug Designation by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) and is currently being administered under a compassionate use programme in France, called Autorisation Temporaire d’Utilisation Nominative (ATUn).
We have developed a capsule formulation of this lead biotherapeutic, called MaaT033. The capsule formulation is designed to address several different tumour indications where ambulatory and long‑term use is necessary, while also maintaining the high, consistent richness and diversity of microbial species. The capsule is currently being evaluated in a Phase I trial in patients with acute myeloid leukaemia or high-risk myelodysplastic syndrome following intensive chemotherapy.
Fermentation technology
As a next step towards transitioning from native to designed off-the-shelf, standardised, microbiome‑based products, we are developing a fermentation technology that allows for the creation of next-generation full-ecosystem biotherapeutics. By growing hundreds of species together, a balanced ecosystem state can be achieved before being administered to the patient, this increases the potential for good bacteria to flourish and therefore restore a fully functional system. While single strain bacteria have been fermented for decades to produce proteins like insulin, the ability to co-ferment a full ecosystem is a groundbreaking step in being able to develop complex microbiome-based therapies that mirror the complex functionalities of the microbiome within the host.
The ability to co-ferment a full ecosystem is a groundbreaking step in being able to develop complex microbiomebased therapies that mirror the complex functionalities of the microbiome within the host”
Based on healthy donor’s gut microbiota and leveraging GutPrint®, we can ferment specific and controlled microbial ecosystems at a very large scale. In the future, these could also be tailored by enriching or depleting the original culture with specific microbes. The ability to manipulate individual species within a specific ecosystem has tremendous advantages; for instance, a positive response to cancer treatment with checkpoint inhibitors was seen when there was a relative abundance of specific microbial strains in patients, in addition to the richness and diversity of the microbial ecosystem. Due to the complexities of inter-species interactions, specific strains are also more likely to successfully colonise the host if they originate as part of a complex ecosystem.
The disruptive technology reinforces our full ecosystem approach and supports expansion to new indications. This technology will also circumvent the need for repeated donor collection campaigns and make the products easily and quickly accessible to a large number of patients.
Regulatory outlook
A challenge in the near term, as the field matures into later stages of clinical development, can be navigating the regulatory processes related to different microbiome-based products. Regulatory agencies are now better equipped to concurrently develop and evaluate guidelines for the microbial sciences and are working closely with companies to bring safe and effective products to patients. The current regulation and classification of microbiome-based therapies in Europe falls under the purview of each respective member state’s competent authority and there is no clearly defined pan-European regulatory pathway for companies that are developing industrially produced intestinal microbiome whole ecosystem-based therapeutics. Notably, MaaT Pharma, Caelus Health, EnteroBiotix and Ferring Pharmaceuticals have joined forces under the umbrella of the Pharmabiotic Research Institute (PRI) within the Intestinal Microbiome‑based Medicines European Task Group (IMM-ETG) to propose a harmonised, pan-European regulatory approach and to obtain a universally accepted product classification for these products.
Overall, microbiome-based therapeutics are a complex, yet promising treatment modality and the field continues to mature with a clearer understanding of the interactive microbial ecosystems that we harbour. Translating that to a clinical setting has the potential to change many patients’ lives.
About the author
Hervé Affagard is an entrepreneur specialised in the healthcare sector. He founded MaaT Pharma in late 2014 together with onco-haematologist Professor Mohammad Mohty and Microbiologist Dr Joël Doré after a professional career that spanned multiple industries. Hervé has led MaaT Pharma’s development from its early concept in 2013 and has been at the forefront of the development of the microbiome healthcare ecosystem in France and Europe. Today, MaaT Pharma is a patientcentric, oncology-focused microbiome company with two full-ecosystem live biotherapeutic programmes in clinical development, 90 patients treated to date in three indications and a total of €38 million raised in dilutive and non-dilutive financing, relying on a diverse, committed 30-people team with expertise ranging from artificial intelligence to microbiology, immunology and oncology. Hervé is also a board member for the French biotech industry association, FranceBiotech.
Issue
Related topics
Biopharmaceuticals, Clinical Development, Clinical Trials, Drug Development, Microbiomes, Regulation & Legislation, Therapeutics
Related organisations
Caelus Health, EnteroBiotix, European Medicines Agency (EMA), Ferring Pharmaceuticals, Intestinal Microbiome‑based Medicines European Task Group (IMM-ETG), MaaT Pharma, US Food and Drug Administration (FDA)
Related drugs
Related diseases & conditions
Autoimmune diseases, Cancer, Clostridium Difficile, Diabetes, Graft versus host disease









