FDARA implementation guidance for paediatric studies of molecularly targeted oncology drugs
Posted: 2 August 2021 | Sanyam Gandhi (Takeda Pharmaceuticals) | 1 comment
This article addresses early planning for paediatric evaluation of certain molecularly targeted oncology drugs, including biological products, for which original New Drug Application (NDAs) and Biological License Application (BLAs) are expected to be submitted to FDA, in accordance with The FDA Reauthorization Act (FDARA).

Early paediatric evaluation of certain molecularly targeted oncology drugs is expected to accelerate the creation of an informed paediatric development plan and ultimately the development of promising drugs for paediatric patients. New Guidance entitled “FDARA Implementation Guidance for Paediatric Studies of Molecularly Targeted Oncology Drugs” was issued on 13 December 2019 and finalised certain material related to implementation of The FDA Reauthorization Act (FDARA) that was included in the draft guidance entitled “Paediatric Study Plans for Oncology Drugs: Questions and Answers” issued on 16 January 2020. Accordingly, the US Food and Drug Administration (FDA) does not intend to finalise the draft guidance entitled “Paediatric Study Plans for Oncology Drugs: Questions and Answers,” which is now withdrawn.
FDA programmes for paediatric oncology
FDA has a dedicated Oncology Center of Excellence that seeks to create a unified and collaborative scientific environment to advance the development and regulation of oncology products for patients with cancer. Its overall mission is to achieve patient-centred regulatory decision-making through innovation and collaboration. The Center helps to expedite development of medical products for oncological malignancies for clinical evaluation.
Acceleration of paediatric development plans for novel anti-cancer agents can be greatly facilitated by transparency of industry sponsors on their individual plans to fulfil European Union (EU) and US requirements”
The Oncology Centre of Excellence has a Paediatric Oncology Program which promotes the development of safe and effective new drugs and biologics to treat cancer in children. The Paediatric Oncology Program holds Paediatric Oncology Product Development Early Advice Meetings with sponsors to discuss paediatric development plans. It also holds various meetings with stakeholders, including the Paediatric Subcommittee of the Oncologic Drugs Advisory Committee, the Oncology Subcommittee of the Paediatric Review Committee (PeRC), as well as other public outreach meetings.
There are many other supportive paediatric legislative initiatives which support mission of the Paediatric Oncology Programme, including the:
- 1997 The Paediatric Exclusivity provision – created in the Food and Drug Administration Modernization Act (FDAMA)
- 2002 Best Pharmaceuticals for Children Act (BPCA) – reauthorisation of the Paediatric Exclusivity provision
- 2003 The Paediatric Research Equity Act (PREA) – a requirement which allows the FDA to require paediatric studies in drugs and biologics for certain applications
- 2007 Re-authorization of BPCA and PREA in the Food and Drug Administration Amendments Act (FDAAA)
- 2010 The Biologics Price Competition and Innovation Act of 2009 (BPCI) that was included in the Patient Protection and Affordable Care Act – it created a framework for the approval of follow-on biologics and made biologics, including follow-on biologics, eligible for paediatric exclusivity through amendment of section 351 of the Public Health Services Act. BPCI sunsets in March 2015
- 2012 BPCA and PREA made permanent in the Food and Drug Administration Safety and Innovation Act (FDASIA)
Collaboration between FDA and EMA
The FDA and European Medicine Agency (EMA) recently published guidance in March 2021 about alignment on oncology paediatric development plans for international clinical trial collaboration necessitated by small study populations in rare diseases such as childhood cancer. Acceleration of paediatric development plans for novel anti-cancer agents can be greatly facilitated by transparency of industry sponsors on their individual plans to fulfil European Union (EU) and US requirements and by simultaneous submission of initial paediatric study plan (iPSPs) and paediatric investigation plans (PIPs) to the FDA and EMA, respectively. A new Common Commentary Template was issued from both health authorities. This template adequately addressing these issues upfront will permit focused discussions during cluster calls, allowing for co-ordination of global development plans. It covers development plans, timelines and comparison of both health agencies from the prospect of non-clinical, clinical and quality prospective.
Molecular targeted therapy of cancer
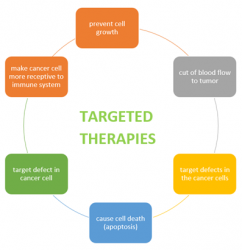

Figure 1: Mode of actions for targeted therapies.
Surgery, radiation and chemotherapy are common therapies for major cancer treatments, but molecularly targeted therapy gives much higher precision. It works on a cellular level by genetically altering cancer cells that are essentials to tumour development and survival. These specific agents work by targeting different signalling pathways. For example, Sorafenib (a multi-kinase inhibitor); dovitinib (a receptor inhibitor) are approved agents that target the vascular endothelial growth factor receptor (VEGFR) via the Ras/Raf/MEK/ERK pathways and target hepatocellular carcinomas.1,2
Paediatric molecular target list
The FDA has issued two lists to help industry for planning and developing paediatric study plan as per the FDARA and Paediatric Research Equity Act:
- Relevant molecular targets – molecular targets for which existing evidence and/or biologic rationale exist to determine their potential relevance to the growth or progression of one or more paediatric cancers. Eg, gene abnormality (ABL1/2, ALK, BRAF), cell lineage (AKR1C3, BCOR, CD19), tumour microenvironment and immunotherapy (IFNgamma IL-2 LAG3)
- Non-relevant molecular targets – targets for which there is evidence that they are not associated with the growth or progression of paediatric tumours for which requirement for early paediatric evaluation of drugs and biologics which are directed at these targets would be waived. Eg, Automatic Waivers; AR ESR1 ESR2 GnRHR PSA/PSCA/PSMA
Final thoughts
In conclusion, FDA is encouraging participation with international meetings, sponsors, investigators, patients advocates and regulators to discuss development strategies for specific paediatric cancer in the context of the number of investigational drugs available for assessment and the highly variable unmet medical needs of distinct paediatric populations with specific childhood cancers. New amendments to section 505B of the Federal Food, Drug and Cosmetic Act provide all elements of early planning of paediatric evaluation of certain molecularly targeted oncology drugs for which original New Drug Applications (NDAs) and Biologics License Applications (BLAs) are expected to be submitted to the FDA.
About the author
Sanyam Gandhi, Regulatory Affairs Strategist, Takeda Pharmaceuticals, Cambridge, Massachusetts, US.
Sanyam is working as Regulatory Affairs Strategy Lead in Takeda pharmaceuticals in Cambridge, US. He has a bachelor’s and master’s in pharmacy from India and extensive experience in the field of pharmaceuticals and regulatory affairs. He completed certification programs at Harvard University and the Regulatory Affairs Professionals Society, both US.
Sanyam has worked with many major pharmaceutical companies including GSK, TEVA, Catalent, Ranbaxy and Shire in multiple countries and is an active member in the different pharmaceutical associations for example TOPRA (The Organisation for Professionals in Regulatory Affairs, UK), RAPS (Regulatory Affairs Professionals Society, US), Royal Pharmaceutical Society, UK, and Pharmacy Council of India (PCI).
He has published many scientific research and review articles, as well as books on different pharmaceutical subjects, but regulatory affairs is area of his excellence. He is also Editor for the International Journal of Pharmaceutical and Biological Science; International Journal of Medical and Biomedical Studies; International Journal of Medical Studies and Clinical Research; and Journal of Drug Discovery and Therapeutics (JDDT).
References
- Antoniou EA, Koutsounas I, Damaskos C, Koutsounas S. Remissiodn of psoriasis in a patient with hepatocellular carcinoma treated with sorafenib. In Vivo, 30 (2016), pp. 677-680
- Cheng AL, Thongprasert S, Lim HY, et al. Randomized, open-label phase 2 study comparing frontline dovitinib versus sorafenib in patients with advanced hepatocellular carcinoma. Hepatology, 64 (2016), pp. 774-784









Very informative