Radiomolecular precision oncology: a comprehensive treatment approach with high potential
Posted: 21 February 2022 | Richard Baum (ICPO) | No comments yet
A significant obstacle still faced in oncology is damage to healthy cells inflicted during treatment, which robs patients of their quality of life while battling cancer. Targeted radionuclide therapy (TRT) is a type of radiomolecular precision oncology that addresses this issue by deploying cancer-killing radiation directly to tumour cells, with little damage to healthy, surrounding tissue. Richard Baum, President of the International Centers for Precision Oncology (ICPO) Academy, explains more.

Despite great therapeutic advances, cancer remains a leading cause of death worldwide. A major limitation in the treatment for most cancers is the inability of therapies to effectively target cancer cells without damaging normal ones. Precision diagnosis and treatment providing a personalised approach is the new focus for patients with cancer. An exciting, novel approach, targeted radionuclide therapy (TRT) has emerged as a novel avenue to specifically target cancer cells. TRT is very different from traditional radiation therapy, which uses external beam radiation to destroy cancer cells. TRT, a form of radiomolecular precision oncology, uses a sophisticated targeting approach that keeps patient wellbeing at the forefront of therapy, from diagnosis to treatment.
A new approach to targeting cancer
At the heart of TRT is a new class of drugs called radiopharmaceuticals, which deliver radiation directly to cancer cells. Radiopharmaceuticals consist of a specific cancer-targeting molecule (eg, a peptide or antibody) combined with a radioisotope, which kills the cells. The radiopharmaceutical (the ‘key’) is injected intravenously and circulates through the bloodstream until it binds to the tumour‑specific receptor or enzyme (the ‘lock’). Once attached to the cancer cells (see Figure 1), the radioisotope emits ionising radiation that damages DNA, resulting in cancer cell death and halted tumour growth, with little damage to the normal surrounding tissues. Given this highly precise localisation, TRT is rapidly emerging as a high‑potential radiation oncology treatment for patients with cancer who have limited treatment options.
Transforming cancer diagnosis with precision localisation
In the case of patients with metastatic cancer, accurate and sensitive imaging enables the early detection of cancers that would otherwise spread rapidly and silently, presenting late with advanced disease. Radioisotopes in conjunction with highly sensitive molecular imaging technologies such as PET (positron-emission tomography) or SPET (single‑photon emission tomography) help to visualise tiny deposits of cancer cells in affected organs that are otherwise too small to be detected by conventional imaging like computerised tomography (CT) or magnetic resonance imaging (MRI).
If you can see it, you can treat it
An attractive feature of TRT is that the targeting molecule can typically be used interchangeably for diagnosis and therapy, requiring only a change of the medical radioisotope. This affords doctors an early impression of whether the treatment will work. Usually, a radioisotope with a short half-life is used for diagnosis, while those with longer half‑lives are used for therapy. Radiation is emitted either as alpha or beta particles, or gamma rays. Alpha particles (high charge, low penetration power) and beta particles (lower charge, higher penetration power) are typically used for treatment and positron emission/gamma rays (no charge, high penetration) are used for diagnosis. TRT therefore enables the concept of ‘theranostics’ (therapy and diagnostics), which is guided by the founding principle: if you can see it, you can treat it.
The potential of radiomolecular medicine
As a rising star in precision oncology, TRT is redefining the concept of radiomolecular (previously named “nuclear”) medicine, enabling it to reach its full potential by improving patient outcomes while supporting quality of life. In cancer, where treatment is often limited by advanced disease, TRT presents an attractive opportunity to deliver targeted and precise treatment to patients who otherwise have very limited treatment options. This treatment method is already being used in indications such as neuroendocrine tumours of gastroenteric, pancreatic (GEP-NETs) or lung origin.1 Due to their asymptomatic nature, these types of neoplasms often go undetected for prolonged periods of time until they have metastasised, making surgical cure impossible. Although GEP‑NETs are classified as rare cancers,2 the number of people living with this disease is quite high, with numbers rising year-on-year.3 For these patients, TRT stands as a new approach for effectively delivering medication addressing an otherwise unmet need.
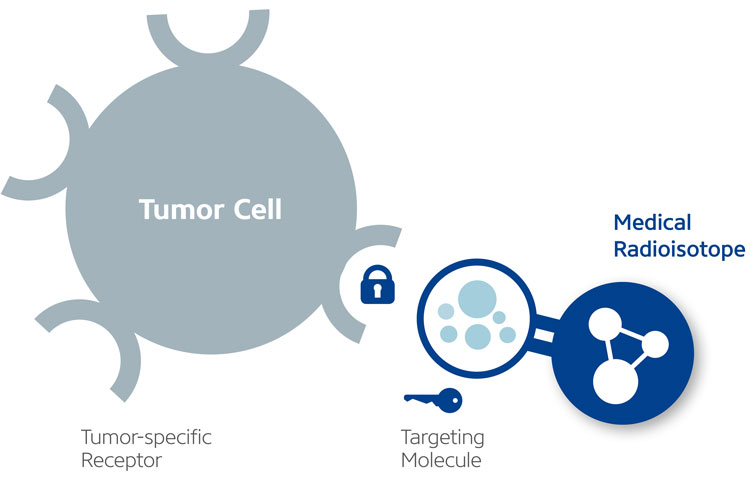

Figure 1: The key-and-lock principle (reference 10)
The rarest drug on Earth: supply and demand of radioisotopes
To fully harness the power of targeted radionuclide therapy, the production and supply of medical radioisotopes is critical. This is particularly true for rare radioisotopes such as actinium-225, sometimes referred to as “the rarest drug on earth”. Although limited amounts of the radioisotope are readily available, actinium’s promise is exponential. Companies are working hard to achieve the means and expertise to produce it effectively and efficiently. Rare or not, all medical radioisotopes must not only be produced and supplied quickly to meet a rising global patient population, but they must also adhere to strict quality control measures. Obtaining highly pure, contaminant-free radioisotopes is imperative to provide patients with top quality diagnoses and treatments. From research, production and supply, and all the way to drug development, TRT is receiving heightened attention.
Late-stage clinical progress and successes
TRT has been investigated in various clinical trials that are making waves in the industry and have yielded very promising results. In 2013, radium-223-dichloride (Xofigo) was approved as the first alpha emitter to treat painful bone metastases of prostate cancer. Approvals for other radiopharmaceuticals followed in recent years. In 2018, for instance, lutetium-177-dotatate (Lutathera) was approved for the treatment of various neuroendocrine tumours (NETs) affecting mainly the digestive tract. Further candidates are in clinical development: Germany-based ITM Isotope Technologies Munich SE, for example, is conducting two pivotal Phase III clinical trials, COMPETE4 and COMPOSE,5 exploring the TRT approach with n.c.a. lutetium-177-edotreotide in patients with GEP‑NETs. Both trials build on promising Phase II data that demonstrated improved progression‑free survival, a low uptake by normal organs and a high tumour‑to-kidney ratio.6 Additionally, Novartis has published positive results from a Phase III trial called VISION, which studied TRT with lutetium-177‑PSMA-617 in patients with advanced, metastatic prostate cancer. It is expected that this treatment will become available for prostate cancer patients this year. Due to its emerging success, entire conferences are now dedicated to TRT, including the Theranostics World Congress7 and the International Centers for Precision Oncology (ICPO) Forum, which bring together international experts and patient advocacy representatives to discuss novel developments in this rapidly developing field.
A new era of personalised medicine
With its ability to selectively destroy cancer cells and even bulky tumours while giving patients a high quality of life during treatment, TRT poses an exciting new avenue in the field of personalised medicine. It holds the potential to unlock multiple treatment channels utilising an array of targeting methods and medical radioisotopes. For example, new radiopharmaceuticals are being developed to target the fibroblast activation protein (FAP), which is highly expressed and detectable in the majority of epithelial cancers, including over 90 percent of breast, lung, colorectal and pancreatic carcinomas.8 Following on from beta-radioisotopes, targeted alpha therapy provides highly localised, powerful radiation which is particularly lethal to cancer cells. This versatility has led to companies being founded specifically around TRT.9 Targeted radionuclide therapy has ushered in a new era of precision oncology and personalised medicine. This multifaceted therapeutic modality is on an accelerated path forwards and is providing new therapeutic approaches to patients with cancer who have limited treatment options.
PROF. DR. MED. Richard Baum is President of the International Centers for Precision Oncology (ICPO) Academy, Board member of the ICPO, and Consultant of the Advanced Center for Radio-molecular Precision Oncology at Curanosticum Wiesbaden-Frankfurt. He performed the first radioimmunotherapy and peptide receptor therapy in Germany and the first 177Lu-based prostate-specific membrane antigen radioligand therapy globally. Richard has treated over 2,000 patients with more than 7,000 PRRT applications. He has received accolades including the Saul Hertz Award, a prestigious nuclear medicine lifetime award. An avid supporter of theranostics with professorships around the world, Richard contributes greatly, clinically and academically, to nuclear medicine.
References
- Malcolm J, Falzone N, Lee B, Vallis K. Targeted Radionuclide Therapy: New Advances for Improvement of Patient Management and Response. Cancers [Internet]. MDPI AG; 2019 Feb 25;11(2):268. Available from: http://dx.doi.org/10.3390/cancers11020268
- 2. Oronsky B, Ma P, Morgensztern D, Carter C. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia [Internet]. 2017;19(12):991-1002. Available from: https://www.sciencedirect.com/science/article/pii/S1476558617303470
- Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncology [Internet]. 2017;3(10):1335. Available from: https://pubmed.ncbi.nlm.nih.gov/28448665/
- Efficacy and Safety of 177Lu-edotreotide PRRT in GEP-NET Patients – Full Text View – ClinicalTrials.gov [Internet]. Clinicaltrials.gov. 2022 [cited 19 January 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT03049189
- Lutetium 177Lu-Edotreotide Versus Best Standard of Care in Well-differentiated Aggressive Grade-2 and Grade-3 GastroEnteroPancreatic NeuroEndocrine Tumors (GEP-NETs) – COMPOSE – Full Text View – ClinicalTrials.gov [Internet]. Clinicaltrials.gov. 2022 [cited 19 January 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT04919226
- Baum R, Kluge A, Kulkarni H, et al. [177Lu-DOTA]0-D-Phe1-Tyr3-Octreotide (177Lu-DOTATOC) For Peptide Receptor Radiotherapy in Patients with Advanced Neuroendocrine Tumours: A Phase-II Study. Theranostics. 2016;6(4):501-510. doi: 10.7150/thno.13702
- Theranostics World Congress [Internet]. Twc-2022.org. 2022 [cited 19 January 2022]. Available from: https://www.twc-2022.org/
- Altmann A, Haberkorn U, Siveke J. The Latest Developments in Imaging of Fibroblast Activation Protein. Journal of Nuclear Medicine [Internet]. 2020;62(2):160-167. Available from: https://jnm.snmjournals.org/content/62/2/160
- Radiopharmaceutical Market Shows Huge Demand and Future Scope Including Top Players 2031 | Medgadget [Internet]. Medgadget.com. 2021 [cited 8 December 2021]. Available from: https://www.medgadget.com/2021/10/radiopharmaceutical-market-shows-huge-demand-and-future-scope-including-top-players-2031.html 10. ITM – Targeted
Issue
Related topics
Related organisations
International Centers for Precision Oncology (ICPO), Thermo Fisher Scientific









