Towards ‘smart’ vaccine development and manufacturing
Posted: 24 August 2022 | Soundar Kumara (Pennsylvania State University), Vijay Srinivasan (NIST), Vishnu Kumar (Pennsylvania State University) | No comments yet
The coronavirus pandemic encouraged biopharmaceutical companies to adopt smarter approaches to vaccine development. Here, Vishnu Kumar and Soundar Kumara from Pennsylvania State University, and Vijay Srinivasan from National Institute of Standards and Technology (NIST), explore the emergence of platform-based vaccine technologies and their potential expansion to treat other life-threatening diseases.


THE GLOBAL outbreak of the COVID-19 pandemic triggered an urgent need to protect people’s lives and livelihoods and the healthcare community recognised quite early that vaccines were the best solution to this crisis. However, the biopharma industries realised that traditional vaccine development and manufacturing techniques, which take around five to 10 years for initial development to large-scale distribution,1 were inadequate to meet the growing demand for COVID-19 vaccines. This motivated the biopharma sector to devise smart approaches to develop and manufacture vaccines without compromising on their safety and efficacy.2
Novel biotechnology platforms for vaccine development
Vaccines based on the two novel biotechnology platform-based techniques mRNA (messenger RNA) and viral vector, have proven successful in controlling the spread of the COVID-19 pandemic. It is worthwhile noting that these platform-based vaccines do not involve the injection of pathogens into the human body, as with traditional vaccines, but instead integrate the genetic sequence information of the disease-causing pathogens.2 Owing to their inherent characteristics of being robust and flexible, the platform-based smart vaccine development technique has gained much popularity among biopharma companies. According to the latest estimates from the World Health Organization (WHO), there are more than 80 biotechnology platform-based COVID-19 vaccine candidates in various stages of clinical development.3
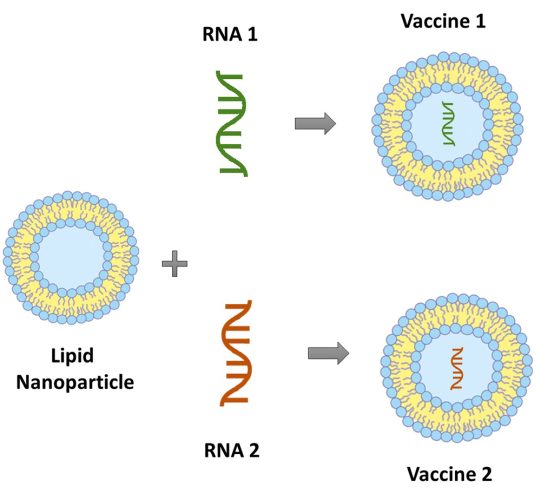

Figure 1: Two different modules (RNA sequences) are combined with the same base platform carrier (lipid nanoparticle) to form two different mRNA platform-based vaccines.
The vaccines developed using the platform technology share a set of components or parts that is common across all the vaccine products derived from that particular platform.4 For instance, vaccines derived from the mRNA platform have a ‘base platform carrier’, usually lipid nanoparticles, that is common across all mRNA vaccine products. When individual components known as ‘modules’ are plugged into this base platform carrier, a distinct vaccine product belonging to the mRNA vaccine platform family is derived.2 The modules are usually mRNA strands, which are tailor-made based on the target disease. The base platform carrier (lipid nanoparticles) envelops the module components (mRNA strands) to generate the required vaccine. As illustrated in Figure 1, when a module component (RNA 1) is embedded to a base platform carrier (lipid nanoparticle), a vaccine product (Vaccine 1) belonging to the mRNA platform is developed. It is interesting to note that by simply swapping the module component to RNA 2, a different vaccine product (Vaccine 2) belonging to the mRNA platform can be generated.
Similarly, in viral vector platform-based vaccines, a modified version of a harmless virus (called a ‘vector’) acts as the base platform carrier and envelops the DNA strands (module). By swapping the DNA strand embedded on the same vector (base platform carrier), a different vaccine can be generated, as illustrated in Figure 2.
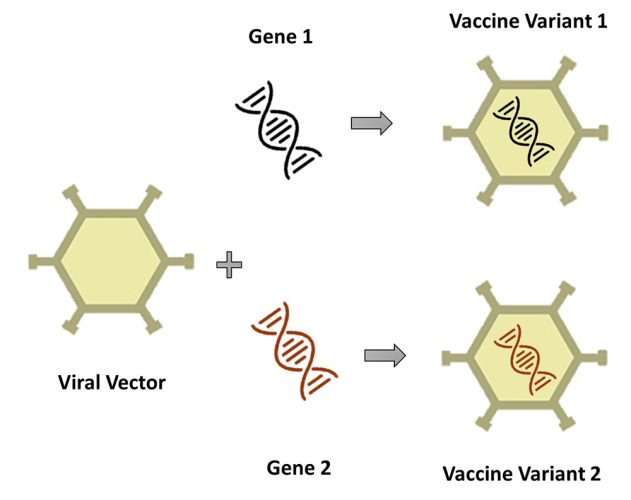

Figure 2: Two different DNA sequences (modules) combined with the same base platform carrier (viral vector) to form two different viral vector vaccine products.
This unique capability of platform-based vaccines makes them highly customisable. Researchers opine that once the genetic sequence of the virus is generated, it can be digitally shared across the globe to accelerate vaccine development. By swapping the genetic instructions (module) of a vaccine to match the new virus (or its variant), and embedding it in the base platform carrier, a new vaccine candidate can be easily developed to tackle the new virus (or its variant). This makes the platform-based vaccines customisable, quick to build and smart.
Digital technologies for smart vaccine manufacturing
Smart manufacturing essentially involves the digitalisation of the manufacturing enterprise, with enhanced connectivity and seamless data and information exchange through closely inter-connected devices and systems.5 The smart manufacturing paradigm can essentially transform the enterprise by improving productivity and customer experience, as well as revolutionising values chains.6 Digital technologies such as Internet of Things (IoT), autonomous robots, augmented reality, cloud computing, etc, lead the current era of digitalisation and facilitate ubiquitous data and information exchange between machines, humans and even enterprises.
In the context of vaccines, smart manufacturing refers to the adoption of smart devices (such as sensors, radio frequency identification [RFID] devices, etc) to generate, process, store and exchange data, and services (including digital tools and applications) to connect these devices during each step of the vaccine manufacturing process. Data collected during each stage, such as temperature, composition, etc, of the vaccine products by the smart devices can be monitored and analysed using cloud-based applications. This can drive informed decisions and strategies to enhance the safety, quality and efficiency of the manufacturing process.
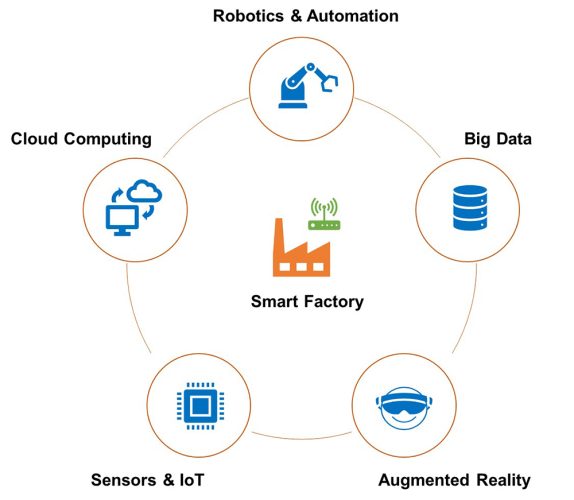

Figure 3: Enabling technologies for making future factories ‘smart’.
Moreover, digital automation tools will be deployed at large scale to complement the efforts to make the vaccine manufacturing process smart. Owing to the safety concerns presented by human-induced contamination and mishandling with vaccine production, interest in adopting autonomous and intelligent devices has grown tremendously in recent times and can be expected to dominate the industry in the future. The recent advancements in augmented reality (AR) technology have created new opportunities for digitally interacting with devices, products and humans using gestures, thereby limiting the need for physical interactions. For example, AR devices can be used to develop touchless applications whereby the human operator can interact with a vaccine product using gestures to obtain its properties.6
Finally, blockchain and similar digital record‑keeping technologies will be adopted to boost the visibility and transparency of the vaccine manufacturing process and supply chain, control counterfeits, and enhance trust among people. This lays the foundation for the development of future smart factories aimed at vaccine manufacturing, as illustrated in Figure 3.
The future of platform-based vaccine technology and manufacturing
The biopharma sector has witnessed significant transformations in recent times. The main driver for this change has been novel platform-based vaccine technologies and smart vaccine manufacturing techniques. The benefit of vaccines based on platform technologies is that they can be easily designed and developed. By embracing digital tools and transformative technologies, the biopharma industries will be well positioned to address the global need for safe and effective vaccines.
Moving forward, biopharma companies are exploring the possible extension of the platform‑based vaccine technology to tackle diseases beyond COVID-19, such as influenza, cancer and AIDS (Acquired Immune Deficiency Syndrome), among others. If this becomes a reality, then it will be possible for us to develop customisable and personalised vaccines to tackle these life‑threatening diseases in the near future.
Disclaimer
Certain commercial systems, products and applications identified in this paper are not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, USA, nor is it intended to imply that they are necessarily the best available for the purpose.
About the authors


Vishnu Kumar is a Doctoral Candidate in Industrial Engineering and a member of the Laboratory for Intelligent Systems and Analytics (LISA) at the Pennsylvania State University, USA. His research interests are in the product design, manufacturing and supply chain domains.


Dr Soundar Kumara is Allen E. Pearce and Allen M. Pearce Professor of Industrial Engineering and the Director of the Center for Applications of AI & ML to Industry (AIMI) at the Pennsylvania State University, USA. His research interests are in data science, graph analytics and AI and ML, with applications in manufacturing and healthcare.


Dr Vijay Srinivasan is currently with the Engineering Laboratory at the National Institute of Standards and Technology, USA. Previously, he was the Chief Standards and Solutions Officer for Product Lifecycle Management (PLM) at IBM. He has also served as an adjunct professor of Mechanical Engineering at Columbia University, USA.
References
- Kumar V, Srinivasan V, Kumara S. 2021. Towards Smart Vaccine Manufacturing: A Preliminary Study During Covid-19. Proceedings of the ASME International Mechanical Engineering Congress and Exposition. https://doi.org/10.1115/IMECE2021-70516.
- Kumar V, Srinivasan V, Kumara S. 2022. Smart Vaccine Manufacturing Using Novel Biotechnology Platforms: A Study During COVID-19. Journal of Computing and Information Science in Engineering. 22(4):040903. https://doi.org/10.1115/1.4053273.
- WHO Landscape of COVID-19 Vaccine Candidates. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed June. 15 2022).
- Hume HK, Lua LH. 2017. Platform technologies for modern vaccine manufacturing. Vaccine. 35(35):4480-5. https://doi.org/10.1016/j.vaccine.2017.02.069.
- Lu Y, Morris K, Frechette S. Current Standards Landscape for Smart Manufacturing Systems. NIST Interagency/Internal Report (NISTIR). 2016. https://doi.org/10.6028/NIST.IR.8107.
- Chircu AM, Sultanow E, Sözer LD. A reference architecture for digitalization in the pharmaceutical industry. INFORMATIK. 2017. https://dx.doi.org/10.18420/in2017_205.
Issue
Related topics
Big Data, Biologics, Blockchain, DNA, Drug Development, Drug Manufacturing, Industry Insight, Informatics, Internet of Medical Things (IoMT), mRNA, Research & Development (R&D), Vaccine Technology, Vaccines, Viruses









