Pharmaceutical manufacturing – for now and the future
Posted: 7 April 2008 | Per Vase, Specialist, NNE A/S | No comments yet
The pharmaceutical industry has, for many years, operated in a special environment with strong regulation and patent protection. Production efficiency and yields have not, as in many other industries, been the major competition parameter and, as a result of this, pharmaceutical manufacturing has a low manufacturing performance compared to other industries.
The pharmaceutical industry has, for many years, operated in a special environment with strong regulation and patent protection. Production efficiency and yields have not, as in many other industries, been the major competition parameter and, as a result of this, pharmaceutical manufacturing has a low manufacturing performance compared to other industries.
The pharmaceutical industry has, for many years, operated in a special environment with strong regulation and patent protection. Production efficiency and yields have not, as in many other industries, been the major competition parameter and, as a result of this, pharmaceutical manufacturing has a low manufacturing performance compared to other industries.
Current state: Quality by Inspection
A famous article in The Wall Street Journal expressed it this way: “pharmaceutical manufacturing techniques lag far behind those of potato-chip and laundry-soap makers.”i In order to avoid defective products reaching the market heavy Quality Assurance (QA) and Quality Control (QC) strategies have been established. A recent study by IBMii shows that pharmaceutical manufacturing typically has a process sigma leveliii of 2.5 in production corresponding to a Cpiv of 0.83 or 150000 ppm defects. In comparison, release has a quality sigma level of 5 corresponding to a Cp of 1.67 or 200 ppm defects. No other industry has this 3 orders of magnitude defect difference between produced quality and released quality. It is the result of an incredible effort in QA and QC, especially in end-product testing and sorting, leading to Quality by Inspection. This is carried out to absolute perfection and there is not more to gain following this route. However, the following are two drawbacks to this working practice:
- It drives the prices up, due to high Costs of Poor Quality (CoPQ)
- It makes it impossible to improve the released quality even further
The relations between Sigma level, Yield, Cp, system downtime and CoPQ are shown in Table 1.
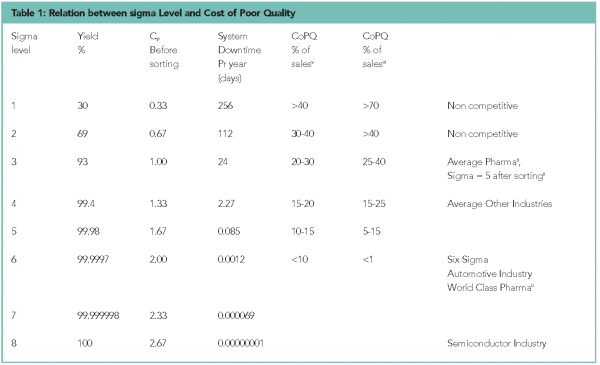

The high CoPQ within pharmaceutical manufacturing is now a focus area for the regulatory authorities, as stated in the FDA Process Analytical Technology (PAT) Guidancevii: “The health of our citizens depends on the availability of safe, effective and affordable medicines”. The pharmaceutical industry has to find a more efficient way of controlling manufacturing processes to make medicines affordable for a larger group of customers. In addition, the quality needs to be improved further; 200 ppm is not good enough for critical characteristics. The industry cannot continue to increase QC efforts by even larger sample sizes in end product testing. This general industry trend can be seen in the latest ISO sampling standard ISO21247, which moves away from the traditional AQL (Acceptable Quality Level) sampling methods and recommends screening (continuous monitoring) instead for critical characteristics. This is because a realistically sized sample plan has an AQL in the order of 0.1-1 per cent, but the acceptable failure rate is in the order 1 ppm for critical characteristics. Sampling does not work for such low acceptable failure rates.
Future state: manufacturing excellence
The first step from quality by inspection to manufacturing excellence is to remove unnecessary production steps and thereby reduce the number of defect opportunities. This could be achieved by the application of Lean tools. The next step is to increase the capability of the remaining necessary production steps. This should be done by increasing process understanding using, for example, process analysers, Design of Experiments (DoE) and chemometrics. This would make it possible to monitor and adjust processes in-line, keeping quality at a constant high level, despite unavoidable variation in raw materials and process conditions leading to quality by control. This requires the use of, for example, advanced process control systems and Statistical Process Control (SPC). The final step is to increase process understanding to a level where robustness can be built in through proper design of products and processes, thus minimising the need for process monitoring and adjustment. Hereby we will have reached quality by design.
A lot can be learned by looking at how this has been done in other industries. PAT has a lot of similarities with Six Sigma and the pharmaceutical industry should learn from the experience of implementing Six Sigma in other industries. The situation within the pharmaceutical industry is not different to the situation within the electronic or automotive industry 20 years ago. They were able to make the transformation from quality by inspection to quality by control and quality by design. Six Sigma cost savings models from variance reduction can be used to quantify PAT benefits as shown in Table 1. Another important lesson learnt from Six Sigma is that the statistical tools such as DoE and SPC should be used by the process users and not by statistical experts. One area in which the pharmaceutical industry is different from the automotive and electronic industry is the process complexity requiring the need of advanced process analysers, typically spectrometers, to be able to monitor processes. Spectral information is characterised by an enormous amount of data, typically information at hundreds or even thousands of wavelength intervals. To be able to analyse this large an amount of data in-line, a larger tool-box than is typically used within Six Sigma is needed. However, again we can learn from other industries, e.g. food and petro-chemical. They have for years used chemometrics tools to reduce the dimensionality of data allowing fast in-line analyses of the large amounts of correlated spectral data.
How to get to the future state
The current state of manufacturing capability within the pharmaceutical industry is far from the desired state. The industry has to go through a long journey from quality by inspection and scrap to quality by design. However, other industries have shown that it can be done and by learning from them and adapting their tools (Lean, Six Sigma, chemometrics) there is a roadmap that can shorten the journey. Since most of these tools are new for the pharmaceutical industry, the journey should not be performed as one long jump, it has to be performed as a series of small incremental steps in order to learn and become familiar with the tools.
Now let’s get started and take the first steps.
References
- Abboud, Leila and Scott Hensley. “New Prescription for Drug Makers: Update the Plants.” The Wall Street Journal. September 3, 2003
- http://www-1.ibm.com/services/ us/imc/pdf/ge510-4034-metamorphosis-of-manufacturing.pdf
- The number of standard deviations between target value and specification limit
- The ratio between tolerance window and process width
- M.J. Harry, Quality Progress, May 1998, p 60-64
- T.J. Clark, Success Through Quality, Quality Press 1999, ISBN0-87389-441-3, www.successthroughquality.com
- http://www.fda.gov/cder/guidance/ 6419fnl.htm
Per Vase
Specialist, NNE A/S
Per holds a Ph.D. in Materials Science and an MSc in Experimental Physics. He recently joined NNE’s Process Analytical Technology team as a data analysis expert, but has more than 10 years of experience from previous employments in various industries. Per has worked with Six Sigma in New Businesses and has a proven track record of bringing products rapidly from the Research Phase to the Quality Controlled Production Phase by the use of Six Sigma Tools, especially DoE and SPC. Per has a proven track record in combining compliance efforts with process optimisation efforts within the manufacture of Medical Devices. Ensuring both high quality and low costs.