Using alkali-metal cation recognition of α-cyclodextrin to detect lithium ions by MALDI-TOF
Posted: 22 February 2023 | Ahmad Amini, Johan Carlsson | No comments yet
Here, Ahmad Amini and Johan Carlsson from the Swedish Medical Products Agency discuss the use of matrix‑assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF MS) for identification of lithium in pharmaceutical preparations.

Lithium (Li) is the third element in the periodic table that belongs to the group of alkali metals. It was discovered by Johann August Arfwedson in the early 1800s in the mineral petalite. This is thought to explain the origin of the element’s name; from lithos (Greek for stone). It is found as different salts such as sulphate, acetate and chloride. Lithium has nine known isotopes, of which two are stable – 6Li and 7Li, which have abundances of 7.5 percent and 92.5 percent, respectively.1
Lithium at high concentrations is toxic to humans, animals and plants, eg, ingestion of 5g of LiCl can cause fatal toxicity”
Lithium, depending on its concentration or exposure, could be biologically important to living organisms.2,3 Lithium at low-to-intermediate concentration has been used as a therapeutic agent for more than 50 years for the treatment of bipolar disorder.4 However, lithium at high concentrations is toxic to humans, animals and plants, eg, ingestion of 5g of LiCl can cause fatal toxicity.5 Lithium has also demonstrated to be an effective cathionising agent for MALDI-analysis of, eg, natural wax esters, sphingolipids, carbohydrates, lipids and polymers.6-8
Cyclodextrins are a family of cyclic oligomers produced from starch by means of enzymatic digestion.9 The most abundant natural cyclodextrins are α-CD, β-CD and γ-CD, including respectively six, seven and eight glucopyranose units. They present a shape similar to a hollow torus with different polarities in their interior and exterior surfaces; ie, hydrophobic inner cavity and hydrophilic exterior.10
The interior of the cyclodextrins readily associates with various organic molecules to form inclusion complexes.10,11 The hydroxyl groups at the mouth of the CD cavity form supramolecular complexes by hydrogen bonding or electrostatic interactions.12 Such properties enable the formation of host-guest complexation, with inorganic metal salts.13,14 The driving forces behind the host-guest inclusion complexation of the CD and individual guest molecule include electrostatic, hydrogen bonding, van der Waals and hydrophobic interactions.15-17
Soft ionisation techniques, such as MALDI-TOF MS and electrospray ionisation, have provided the possibility of exploring the noncovalent CD complexes.9,18,19
In the present study adduct formation between α-CD and lithium ions has been exploited to detect lithium in pharmaceuticals.
Results and discussion
α-cyclodextrin, like other cyclodextrins such as β-CD, has a high affinity to pair with various alkali metals in the sample matrix to form a variety of adducts. As illustrated in Figure 1 the positive ion mode MALDI analysis of α-CD and β-CD generate ion forms of alkali metal adducts, eg, [α-CD-Li]+, [α-CD-Na]+ and [α-CD-K]+. α-CD (MW = 792.8 g/mol), α-CD as opposed to β-CD, has high aqueous solubility, ie, more than 100 g per litre of water at 25oC. CD is a co-matrix and functions as an alkali metal sink capturing metal ions, ie, Li+, Na+ and K+. This is due to the high affinity of sugars to form alkali-metal ion adducts in mass spectrometry.20
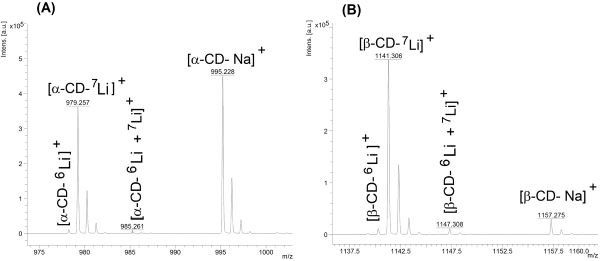

Figure 1: Cationisation of α-CD (A) and β-CD (B) by lithium, sodium and potassium cations. Lithium chloride was dissolved in 50 mg/ml α-CD and 10 mg/ml β-CD at a concentration of 200 µg/ml. Analyses were carried out in the reflectron mode by using ACHCA dissolved in acetonitrile and trifluoro acetic acid as the MALDI matrix.
Association with the alkali metal ions, and thereby formation of the cathionised CD, depends on the affinity of the CD for these metal ions, the CD/metal ratio as well as metal cation properties such as ion radius and electron configuration.13
Therefore, a solution of 50 mg/ml (51mM) α-CD in water was used as solvent to have an excess of α-CD molecules for the adduct formation.
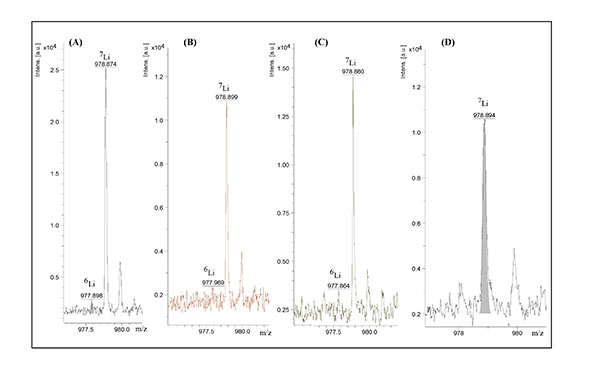

Figure 2: MALDI-TOF MS analysis of trifluoroacetate (A), lithium acetate (B), lithium chloride (C) and lithium sulphate (D). Lithium concentrations were 0.3 µg/ml (A, B and D) and 0.4 µg/ml (C). Other conditions were as those given in Figure 1.
Lithium, due to its smaller radius and high polarising strength, can replace Na+ and K+ in α-CD.
Different lithium salts, ie, lithium sulphate, lithium chloride, lithium acetate and lithium trifluoro acetate, at different concentrations in the range of 10-200 µg/ml dissolved in 50mM α-CD were analysed. As Figure 2 illustrates the lowest concentration that could be detected was less than 0.3 µg/ml.
Lithium, due to its smaller radius and high polarising strength, can replace Na+ and K+ in α-CD.22 The relative abundance ratio of 6Li+ and 7Li+ was determined to be 95.2 ± 1.0% (94-96 percent) and 4.7% ± 1.0% (3-6 percent) from the peak areas (A) or peak intensities by the following formula:
ALi (7 or 6)/(ALi(6)+ALi(7))
The determined values deviate significantly from the reported isotopic ratios, ie, 92.5 percent and 7.5= percent.1,7 It may depend on the competition between 6Li and 7Li, present at much higher concentration, to be ionised. As expected, the 6Li+ isotope is not detected when lithium concentration is close to the limit of detection (LOD). Figure 3 depicts spectrum from MALDI-TOF MS analysis of lithium in a lithium tablet.
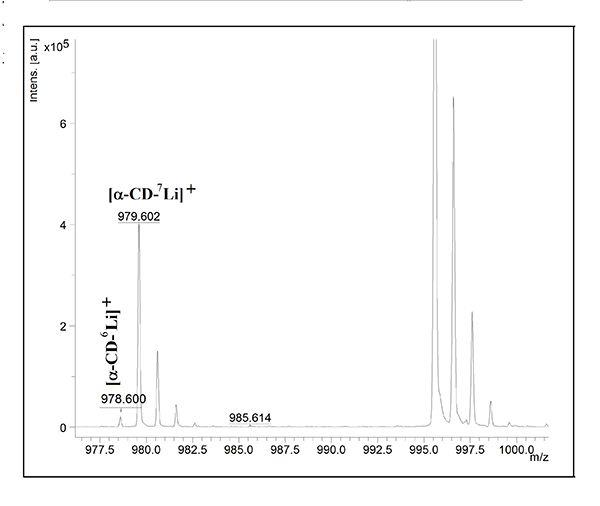

Figure 3: MALDI-TOF MS analysis of lithium in a lithium tablet. The sample was dissolved in 50 mg/ml α-CD. Other conditions were as those given in Figure 1.
Conclusions: MALDI-TOF MS analysis of lithium
Alpha-cyclodextrin doped MALDI-TOF MS provides a fast procedure for the identification of lithium in different analyte samples. The level of LOD was found to be dependent on the complexity of the sample matrix, ie, the LOD increases when sample matrix was involved. The isotopic abundance ratio of 7Li was determined to be 95% ± 1%.
About the authors




Disclaimer: The manuscript presents the personal opinions of the authors and does not necessarily represent the views or policies of SMPA.
References
- Handbook of Stable Isotope Analytical Techniques Vol II 1st Edition – October 17, 2008, Author: Pier de Groot, eBook ISBN: 9780080930114.
- Shahzad B, Mughal M, Tanveer M, et al. Is lithium biologically an important or toxic element to living organisms? An overview, Environ Sci Pollut Res, 2017, 24, 103–115.
- Becker RW, Tyobeka EM, Lithium enhances proliferation ofHL60 promyelocytic leukemia cells, Leukemia Res 1990, 14, 879-884.
- Grunze H, Vieta E, Goodwin G, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Biological Treatment of Bipolar Disorders: Acute and long-term treatment of mixed states
in bipolar disorder, World J Biol Psychia, 2018, 19, 2-58. - Jakobsson E, Argüello-Mirada O, Chiu SW, et al. Towards a Unified Understanding of Lithium Action in Basic Biology and its Significance for Applied Biology, J Membr Biol, 2017, 250, 587-604.
- Cvačka J, Svatoš A. Matrix-assisted laser desorption/ionization analysis of lipids and high molecular weight hydrocarbons with lithium 2,5-dihydroxybenzoate matrix, Rapid Commun Mass Spectrom, 2003; 17,
2203–2207. - Vrkoslav V, Míková R, Cvačka J. Characterization of natural wax esters by MALDI-TOF mass spectrometry, J Mass Spectrom, 2009, 44, 101–110.
- Tran A, Monreal IA, et al. Rapid Detection of Viral Envelope Lipids Using Lithium Adducts and AP-MALDI High Resolution Mass Spectrometry, J Am Soc Mass Spectrom. 2021, 32, 289–300.
- Biwer A, Antranikian G, Heinzle E. Enzymatic production of cyclodextrins, Applied Microbiology and Biotechnology, 2002, 59, 609-617.
- Cid-Samamed A, Rakmai J, Mejuto JC, et al. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications, Food Chem, 2022, 384, 1-14.
- Zhang M, Shi Z, Bai Y, et al. Using molecular recognition of β-cyclodextrin to determine molecular weights of low-molecular-weight explosives by MALDI-TOF mass spectrometry, J Am Soc Mass Spectrom, 2006, 17,
189–193. - Bender ML, Komiyama M. Cyclodextrin Chemistry, Springer-Verlag: New York, 1978, 2–9.
- Dossmann H, Fontaine L, Weisgerber T, et al. First steps to rationalize host-guest interaction between α-, β-and γ-cyclodextrin and divalent first row transition and post-transition metals (subgroup VIIB, VIIIB and
IIB), Inorg Chem Am Chem Soc, 2021, 60, 930-943. - Norkus E, Metal ion complexes with native cyclodextrins. An overview, J Incl Phenom Macrocycl Chem, 2009, 65, 237-248.
- Chen Y, Zou Z, Dai X, et al. Gas-phase complexation of a/b-cyclodextrin with amino acids studied by ion mobility-mass spectrometry and molecular dynamics simulations, Talanta 2018, 186, 1-7.
- Schalley CA. Molecular recognition and supramolecular chemistry in the gas phase, Mass Spectrom Rev, 2001, 20, 253-309.
- Kano K. Mechanisms for chiral recognition by cyclodextrins, J Phys Org Chem, 1997, 10, 286-291.
- Lee JU, Lee SS, Lee S, et al., Noncovalent complexes of cyclodextrin with small organic molecules: Application and insights into host-guest interactions in the gaseous phase and condensed phase, Molecules, 2020, 25, 4048-4076.
- Bruni P, Schürch S. Mass spectrometric evaluation of b-cyclodextrins as potential hosts for titanocene dichloride, Int J Mol Sci, 2021, 22, 9789-9803.
- Lee S, Ahn S, Park S, Oh HB. Characterization of permethylated b-cyclodexterin-peptide noncovalently bound complexes using electron capture dissociation mass spectrometry (ECD MS), Int J Mass Spectrom, 2005, 279,
47-52.









