Reflecting on five years of quality control for nitrosamine impurities
Posted: 29 June 2023 | Dr Mrunal Jaywant (USP India) | No comments yet
Since the US Food and Drug Administration (FDA)’s report on a series of nitrosamine-related impurity drug recalls in 2018, pharma has made great strides in its ability to detect and control for these impurities. Dr Mrunal Jaywant, Vice President of R&D at USP India proposed a collaborative, cross-community approach between stakeholders can overcome remaining challenges.


Based on scientific knowledge and process understanding, manufacturers of drugs and drug substances have a broad awareness of potential impurities – including nitrosamine impurities – that could form during manufacturing or degradation. However, it is difficult to predict exactly how processes could behave and how materials could react under each process conditions. Quality control testing can help manufacturers in monitoring their processes and reduce the risks of potential formation of those impurities, thus minimising disruptive and costly drug recalls.
This challenge is further compounded by increasingly complex drug formulations and global ingredient supply chains that stretch manufacturers’ capabilities in testing and control of impurities.
A reduced global inspections rate in a post-pandemic world further augment the challenge; in 2022 the US Food and Drug Administration (FDA) inspected only six percent of approximately 2,800 overseas manufacturers.1
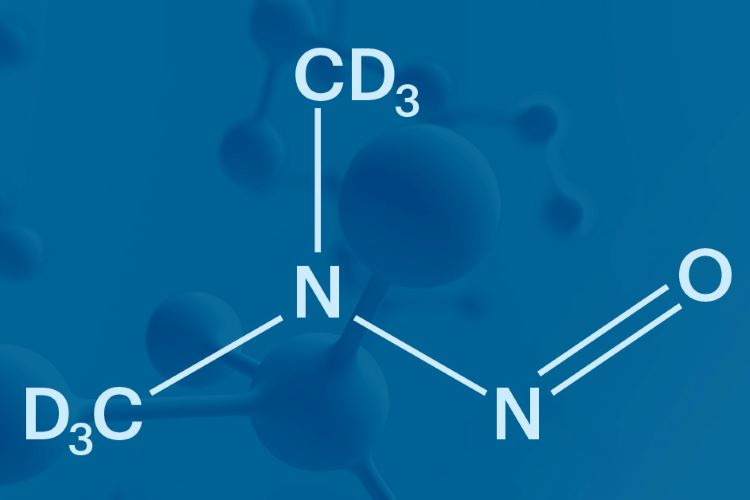

Nitrosamines are not new or unknown impurities.
In 2018, even before the number of global manufacturing inspections fell off precipitously, the industry was alarmed by the FDA’s report that angiotensin receptor blockers (ARBs) containing the active pharmaceutical ingredient (API) valsartan were found to contain nitrosamine impurities that sparked a series of recalls.2
Another ARB affected by the recall, losartan, was at the time, the ninth-most prescribed drug in the US.3 Since that initial recall, more than two dozen specific ARB products have been recalled due to the presence of nitrosamine impurities in selected lots.3
Fortunately, over the past five years, the industry has made great strides in the ability to detect and control for these impurities. Obstacles remain, but with a collaborative, cross-community approach between stakeholders these challenges can be overcome.
First detection of nitrosamine impurities in pharmaceuticals
The first reports of nitrosamines as a known animal carcinogens date back to 1956. Today, nitrosamines are classified by the ICH M7(R1) Guideline as Class 1 impurities, or “known mutagenic carcinogens.”4 But while nitrosamines have been previously reported in food substances and other ingredients, the ARB recalls marked the first time nitrosamine impurities were detected in pharmaceuticals.
the potential problem of nitrosamine impurities in medications may be more widespread than previously thought”
This meant that clear quality guidelines and standards were absent, and manufacturers’ risk assessment for nitrosamines at different stages of ARB’s manufacturing R&D was insufficient. Furthermore, a recent in silico analysis published by United States Pharmacopeia (USP) on more than 12,000 APIs and API-related impurities and degradants from the Global Substances Registration System (GSRS) indicates that the potential problem of nitrosamine impurities in medications may be more widespread than previously thought.5 The USP analysis suggests that as much as 40 percent of APIs and 30 percent of API impurities listed in the GSRS database are vulnerable to formation of nitrosamine impurities.5
Prior to 2018, there were no published nitrosamine limits in Pharmacopoeia monographs. But with the spate of recalls, the FDA developed a Guidance for Industry on Control of Nitrosamine Impurities in Human Drugs.6 This, along with other pharmacopoeial standards, were among the first responses suggested to help identify threats from nitrosamine impurities, protect the global supply chain of medicines, and set the first acceptable intake (AI) limits.
As part of their guidance, global regulatory agencies suggested manufacturers perform risk assessments and conduct multiple analyses to detect impurities. To amplify and ease the implementation of guidance from global regulators, USP responded with a suite of innovative standards, services and solutions.
USP initiatives on nitrosamines
Initially, USP worked with its Expert Committee members to develop and publish Informational General Chapter <1469> on nitrosamine impurities, which has since become official.7 This Chapter provides an outline and approach manufacturers can use to identify sources of nitrosamine impurities, conduct risk assessments, and develop a control strategy. It also provides four analytical procedures for detection and quantification of nitrosamine impurities.7
To support GC <1469> analytical procedures, USP also developed eight highly characterised Reference Standards for testing starting materials, intermediates, solvents and other ingredients in the medicines supply chain for nitrosamines of concern, and other Pharmaceutical Analytical Impurities (PAIs) for identifying small nitrosamines and nitrosamine drug substance-related impurities (NDSRIs).8,9
Complexities of implementation remain as nitrosamines can arise throughout the product life cycle”
Complexities of implementation remain as nitrosamines can arise throughout the product life cycle. This, in turn, has led USP to develop and optimise highly sensitive and robust analytical methods, such as a direct-injection gas chromatography-mass spectrometry/mass spectrometry approach that can detect and quantify six different nitrosamines simultaneously in four commonly used solvents.10
In deploying these science-based standards, USP and its experts also realised that the industry was not necessarily readily equipped with the advanced instrumentation and skillsets required to analyse nitrosamine contamination. In response, USP developed educational resources to support manufacturers and scientists on analytical procedures such as mass spectrometry for monitoring nitrosamine impurities.

USP has also launched the Nitrosamines Exchange – a community hub and virtual platform. In real-time, real-world stakeholders are having conversations about what is happening in their processes, the current challenges they face, and how they are addressing them. In the coming months, USP will publish additional analytical procedures, including those related to excipients, to support testing and controlling quality of medicines.
Today, drug product manufacturers are increasingly performing thorough risk assessments of the potential for formation of small nitrosamines and NDSRIs in their processes and requesting the same from their ingredient manufacturers. Overall, there is much greater awareness of the threat posed by nitrosamine impurities.
drug recalls are also a signal that ‘the system is working’ and that previously unidentified…sources of contamination, like nitrosamines, are being removed from the supply chain”
Based on USP’s experience to date, we can foresee that the identification of new NDSRI and nitrosamine impurities is inevitable.
As drug recalls and withdrawals are undesirable, regulators and standards-setting organisations can provide important services and solutions to prevent them from being necessary. However, it is important to note that withdrawals and recalls are also a signal that ‘the system is working’ and that previously unidentified and undiscovered sources of contamination, like nitrosamines, are being removed from the supply chain, helping to improve patients’ safety.
About the author


References
- A pandemic-fueled drop in FDA inspections of foreign drug plants. [Internet] 2023. [cited 2023June]. Available from: https://www.govexec.com/oversight/2023/04/pandemic-fueled-drop-fda-inspections-foreign-drug-plants/385691/.
- FDA updates and press announcements on angiotensin II receptor blocker (ARB) recalls (valsartan, losartan, and irbesartan). [Internet] US Food & Drug Administration. [cited 2023June]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-angiotensin-ii-receptor-blocker-arb-recalls-valsartan-losartan#:~:text=Update%20%5B10%2F30%2F2018,human%20carcinogen%20(causes%20cancer).
- Gunasekaran PM, Chertow GM, Bhalla V, Byrd JB. Current status of angiotensin receptor blocker recalls. Hypertension. 2019; 74: 1275-1278.
- ICH M7 Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk – Scientific guidelines. [Internet] European Medicines Agency. 2021. [cited 2023June]. Available from: https://www.ema.europa.eu/en/ich-m7-assessment-control-dna-reactive-mutagenic-impurities-pharmaceuticals-limit-potential.
- Schlingemann J, Burns MJ, Ponting DJ, et al. The landscape of potential small and drug substance related nitrosamines in pharmaceuticals. J Pharm Sci. 2023; 112(5): 1287–304.
- Control of Nitrosamine Impurities in Human Drugs – Guidance for Industry. [Internet] Center for Drug Evaluation and Research. US Food and Drug Administration. 2020. [cited 2023June]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/control-nitrosamine-impurities-human-drugs.
- Highlights of general chapter 1469 – Nitrosamine impurities. [Internet] United States Pharmacopeia. 2020. [cited 2023June]. Available from: https://www.usp.org/sites/default/files/usp/document/stakeholder-forum/pnp/highlights-of-1469-nitrosamine-impurities.pdf.
- Nitrosamine reference standards. [Internet] United States Pharmacopeia. [cited 2023June]. Available from: https://go.usp.org/nitrosamines-impurities.
- United States Pharmacopeia. USP expands support for controlling impurities in medicine. Available from: https://www.usp.org/news/usp-expands-support-for-controlling-impurities-in-medicines.
- Kosuri ER, Bhanti M, Jaywant MA, et al. A GC-MS/MS method for trace level quantification of six nitrosamine impurities (NDMA, Ndea, NEIPA, NDIPA, NDPA, and NDBA) in commercially used organic solvents: Dichloromethane, ethyl acetate, toluene, and O-xylene. J Pharm Sci. 2023; 112(5): 1225–30.
Related topics
Biopharmaceuticals, Drug Development, Drug Manufacturing, Drug Safety, Drug Supply Chain, Environmental Monitoring, Impurities, QA/QC, Regulation & Legislation, Research & Development (R&D), Supply Chain, Therapeutics








