Meeting rising demands of a new radiotheranostic era
Posted: 31 August 2023 | Leila Jaafar-Thiel (Nuclidium AG) | No comments yet
Targeted radiotheranostics are on the path to becoming integral to cancer diagnosis and treatment. Their success, however, depends on patient benefit and the ability to meet commercial demands for broader indications. Aside from the therapeutic or diagnostic efficacy, decisions on suitable radionuclide properties and owning the supply chain will be vital to delivering better outcomes, as Dr Leila Jaafar-Thiel, CEO of Nuclidium AG, elucidates.


Cycoltron for production of radionuclides (Credit: Nuclidium)
Cancer patients critically depend on accurate diagnosis and disease treatment. Medical researchers constantly develop innovative therapies and treatment options to improve survival and quality of life. In precision oncology, technological advances have made targeted radiotheranostics a promising option for diagnosing and treating solid tumours. Usually consisting of an injected medical radionuclide and a target molecule, radiopharmaceuticals precisely bind to tumours, destroying tumour cells with a high linear energy transfer from radionuclide decay, thus, halting tumour growth.
By reaching cancer cells that have already spread throughout the body, a targeted radiopharmaceutical treatment offers an alternative for patients with advanced cancer when standard lines of treatment, such as chemotherapy, have failed. In addition, radionuclide therapy has the potential to achieve high efficacy rates since the administered drugs spare surrounding healthy tissue, thus, reducing the burden on the patient. Together with their companion diagnostics for accurate staging, targeted radiotheranostics could offer more comprehensive treatment options for patients with solid tumours.
The development of novel radiotherapeutics ushered in a new era in targeted treatment with the approval of Novartis’ Lutathera in 2018 and Pluvicto in 2022 by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of gastroenteropancreatic neuroendocrine tumours and metastatic prostate cancers, respectively.1-4 Besides these drug products, several companies worldwide are developing radiotheranostics in clinical trials built on various radionuclides. While only lutetium (Lu‑177) is currently clinically established for cancer therapeutics, several radionuclides are suitable for radiopharmaceutical programmes, offering different potential beneficial characteristics. Apart from Lu-177, actinium-225 (Ac‑225) and copper-67 (Cu-67) are the most advanced radionuclides used in radiopharmaceutical development. Radionuclides being tested for diagnostics include gallium-68 (Ga-68), fluorine-18 (F‑18), copper-61 (Cu-61), copper-64 (Cu‑64) and scandium-44 (Sc-44).
Overcoming challenges in patient safety, manufacturing and supply of radiopharmaceuticals
As clinical trials progress and the first results are published, companies’ best candidates will emerge in the next five years. With these results, the industry seeks to understand the differences between radionuclides regarding efficacy and patient safety. Specifically for radiopharmaceuticals, the decay processes of isotopes must be considered in patient safety, as administered radiopharmaceuticals remain in the patient’s body after treatment. Hospital staff must also be protected from radiation while handling the agent. Thus, hospitals must isolate treated patients and implement consistent patient waste management procedures (eg, collecting all patient excreta). This would be a logistical and financial challenge for hospitals, as well as a significant burden for patients, who must remain in complete isolation for a required period of time – the length of which would depend on the nuclide’s half‑life, ie, the time taken for the radioactivity of a specified isotope to fall to half its original activity.
In addition to patient safety and burden, these half-lives and raw materials necessitate manufacturing and delivery processes that challenge radiopharmaceutical companies’ development efforts. Half-lives determine how long radioactivity remains in the patient’s body after treatment and when the produced nuclides must arrive at a hospital for use in patients after manufacturing. The issue of half-lives, availability of radionuclides or other necessary resources in a particular region impact factors such as the duration and length of transport routes. The physical properties of the radionuclide make radiopharmaceutical manufacturing and supply processes highly time critical. An additional consideration for radiopharmaceutical companies when choosing radionuclides for their pipelines are the strict regulatory radiation protection requirements they are subject to.
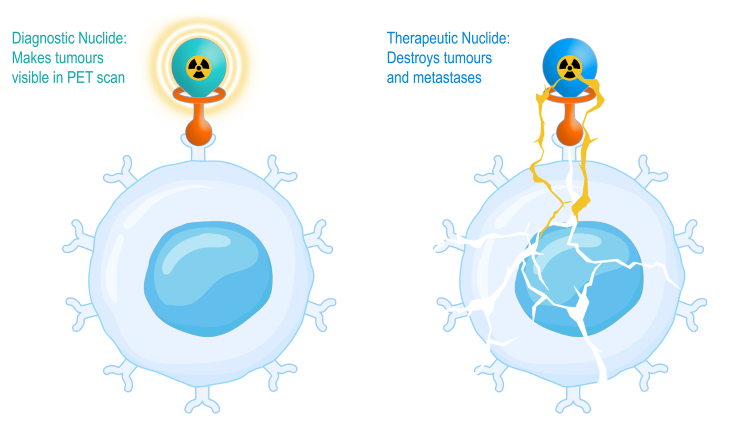

How targeted radiotheranostics work (Credit: Nuclidium)
Critical considerations for radionuclide development efforts
Lu-177, a clinically established radionuclide, is produced by neutron activation of ytterbium-176 (Yb-176) or lutetium-176 (Lu‑176). These stable, highly-enriched isotopes are expensive, scarce earth metals sourced almost exclusively from Russia. Lu-177 is produced mainly in research reactors, some of which are ageing, like the Petten nuclear reactor, and will be decommissioned within 5-10 years. Recently, a programme for activating Lu-177 was started at Bruce Power, a Canadian heavy-water commercial nuclear power plant. The production requires a high thermal neutron flux, typical of heavy‑water reactors, for an adequate production yield and, therefore, cannot be produced in most commercial nuclear power plants, which are either pressurised or boiling water reactors. Irradiation of Lu‑176 also results in Lu‑177m, a by‑product with a half-life of 152 days, which poses a radiation burden for the patient and waste issues for treating hospitals.5
Lu-177 is produced mainly in research reactors, some of which are ageing, like the Petten nuclear reactor, and will be decommissioned within 5-10 years”
Ac-225 is produced in accelerators, thus requiring smaller manufacturing facilities. It, however, relies on comparably large amounts of scarce thorium-232 (Th-232), thorium-229 (Th-229) and radium-226 (Ra‑226). Ra‑226 is radioactive and volatile, while Th-229 is, in part, a by‑product of the nuclear fuel and weapons industries6 and Ac-227 is a by‑product from the spallation of Th-232. Ac-227 cannot be separated from Ac-225 and has a half‑life of 21.77 years, resulting in a high radioactive burden for the patient and waste issues. Long production times and high energy costs in manufacturing are additional considerations.
Copper-based radiopharmaceuticals offer a comparatively easy manufacturing process. Used for diagnostic purposes, Cu-61 is produced via commercial medical cyclotrons and can also be manufactured in accelerators at low yields based on natural nickel. Cu-61’s therapeutic counterpart Cu‑67 is produced in accelerators. One shared characteristic of Cu-61 and Cu-67 is that they require a cheaper and readily-available raw material. For larger commercial batches, highly-enriched material is required, which may be relatively rare and harder to obtain. Cu-617 (T1/2 = 200 min) and Cu-67 (T1/2 = 2.6 days) have a short to medium decay time and decay into stable isotopes; consequently waste management becomes uncomplicated and patient and environmental safety is increased. Compared to the aforementioned copper‑based radionuclides, Cu-64 has a longer production and decay time, which leads to a more expensive production process and a higher radiation burden for the patient. Through its longer half-life of 12.7 hours, Cu-647 is suitable for antibody imaging with slower binding kinetics.
Another radionuclide used in diagnostic imaging is gallium (Ga-68), which is manufactured in either accelerators or radionuclide generators and uses zinc‑68 (Zn-68) or germanium-68 (Ge-68). Ge-68 poses particular challenges as it can only be produced using high-energy accelerators. In addition, Ge-68 itself is a long-lived radionuclide (T1/2 = 271 days) and is a significant contributor to radioactive waste in the supply chain. Due to its short half-life (T1/2 = 68 min), Ga‑68 must be produced in-house and cannot be distributed from a central location.
Until now, fluorine (F-18) has been used extensively in radiodiagnostics and is produced in accelerators from the activation of oxygen-18 (O-18). This nuclide can be transported over longer distances using established transport routes. The complexation of F-18 to a targeting molecule requires a covalent bond in a complex process. Unclear findings and false positives have been recorded using F-PSMA, a metastatic prostate cancer imaging agent.
Innovative approaches to solve radionuclide supply challenges
With pipeline candidates evolving towards commercialisation, environmental safety, manufacturing and supply questions are becoming increasingly important. The rising demand for radiopharmaceuticals has challenged manufacturers and drug development companies to increase production and enhance timely delivery and regional availability for clinical studies and patients worldwide while adhering to regulatory safety protocols. To address these challenges, radiopharmaceutical companies have developed a variety of approaches.
The rising demand for radiopharmaceuticals has challenged manufacturers and drug development companies to increase production and enhance timely delivery and regional availability … worldwide while adhering to regulatory safety protocols”
To meet demands for its Lu-177 and Ac 225-based radiopharmaceuticals, among others, U.S. American Point Biopharma owns proprietary radiopharmaceutical manufacturing facilities and maintains active relations with further manufacturers. For example, in April 2023, the company announced it had entered into a supply agreement with Germany’s Eckert & Ziegler to manufacture Ac-225.8,9 On the other side of the Atlantic, German firm ITM has developed a manufacturing system for producing and transporting its highly pure non-carrier-added Lu-177 safely and efficiently.10 In addition, the company has implemented additional production lines by building new facilities to ensure the availability of radionuclides for patients. Ariceum Therapeutics, another German-based radiopharmaceutical company, partnered with AmbioPharm to support upcoming clinical trials with the necessary supply of radiopharmaceuticals.11 Australia-based Clarity Pharmaceutical partnered with US-based NorthStar Medical Radioisotopes in 2021 to strengthen Cu‑67 supply in the United States and other regions. Switzerland-based Nuclidium partnered with Pharmalogic, a US-based contract development and manufacturing organisation (CDMO) and logistics expert, to consolidate the manufacturing of suitable radionuclides for its copper-based theranostic programme.12 This strategic partnership further ensures the production and clinical supply of Cu-61 for patient diagnosis in North America and aims to accelerate the development of Nuclidium’s theranostic pipeline.
companies must not only use their logistical know-how to knit well-functioning infrastructure networks but also adhere to standards that consider environmental and patient safety”
Radiopharmaceutical companies are making considerable efforts to advance their clinical trials and to ensure smooth manufacturing and delivery processes by building on the potential of their innovative approaches. However, companies must not only use their logistical know-how to knit well-functioning infrastructure networks but also adhere to standards that consider environmental and patient safety. In addition to drug efficacy, treating tumour patients requires a reliable radiopharmaceutical supply and minimal radiation exposure and isolation burden. After all, the task of the companies is to find the best possible treatment options. Owing to its favourable properties, the copper-based approach combines the best possible diagnostic and treatment methods for patients.
Establishing sustainable manufacturing and supply chains
Radiopharmaceuticals can play a significant role in optimising the diagnosis and treatment of solid tumours. The race for the most suitable candidate will be decided not only on the efficacy and patient safety of the drugs developed but also on their environmental safety and availability. It remains critical for companies to establish sustainable manufacturing processes13 and supply chains to ensure the availability of radiopharmaceuticals to any patient in need on demand anywhere in the world.
About the author
Leila Jaafar-Thiel, PhD


References
- European Medicines Agency. Luthathera [Internet]. ema.europa.eu [cited 2023 May]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/lutathera
- European Medicines Agency. Pluvicto [Internet]. ema.europa.eu [cited 2023 May]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/pluvicto
- S Food and Drug Administration. FDA approves new treatment for certain digestive tract cancers [Internet]. ema.europa.eu [cited 2023 May]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-certain-digestive-tract-cancers
- S. Food and Drug Administration. FDA approves Pluvicto for metastatic castration-resistant prostate cancer [Internet]. ema.europa.eu [cited 2023 May]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pluvicto-metastatic-castration-resistant-prostate-cancer
- Konijnenberg MW. Consequences of meta-stable (177m)Lu admixture in (177)Lu for patient dosimetry. Curr Radiopharm. 2015;8(2):145-9.
- Alvarez R. Managing the Uranium-233 Stockpile of the United States. Science & Global Security [Internet], 2; 21:53–69. Available from: https://scienceandglobalsecurity.org/archive/sgs21alvarez.pdf
- van der Meulen N, Talip Z. Non-conventional radionuclides: The pursuit for perfection. Nuclear Medicine and Molecular Imaging. 2022; Vol. 1: 133-142.
- Point Biopharma. Eckert & Ziegler to Supply POINT Biopharma with Actinium-225 [Internet] globenewswire.com [cited 2023 May]. Available from: https://www.globenewswire.com/news-release/2023/04/04/2640620/0/en/Eckert-Ziegler-to-Supply-POINT-Biopharma-with-Actinium-225.html
- Point Biopharma. POINT Biopharma Confirms No Disruptions to the Manufacturing and Clinical Supply for the 177Lu-PNT2002 SPLASH Trial, a Phase 3 Study in Patients with Metastatic Castration Resistant Prostate Cancer (mCRPC) [Internet] globenewswire.com [cited 2023 May]. Available from: https://www.globenewswire.com/news-release/2023/03/07/2621944/0/en/POINT-Biopharma-Confirms-No-Disruptions-to-the-Manufacturing-and-Clinical-Supply-for-the-177Lu-PNT2002-SPLASH-Trial-a-Phase-3-Study-in-Patients-with-Metastatic-Castration-Resistant.html
- ITM Multiplies Production Capacity for its n.c.a. Lutetium-177 [Internet] itm-radiopharma.com [cited 2023 May. Available from: https://www.itm-radiopharma.com/news/press-releases/press-releases-detail/itm-multiplies-production-capacity-for-its-nca-lutetium-177-389/
- Ariceum Therapeutics. Ariceum Therapeutics and AmbioPharm Enter Strategic Manufacturing and Supply Partnership [Internet] ariceum-therapeutics.com [cited 09 2023 May]. Available from: https://ariceum-therapeutics.com/ariceum-therapeutics-and-ambiopharm-enter-strategic-manufacturing-and-supply-partnership/
- NUCLIDIUM and PharmaLogic Form Strategic Partnership for Sustainable Development of Novel Copper-based Theranostics [Internet] nuclidium.com [cited 2023 May]. Available from: NUCLIDIUM and PharmaLogic Form Strategic Partnership for Sustainable Development of Novel Copper-based Theranostics – Nuclidium
- Forsburg CW, Lewis LC. Uses For Uranium-233:What should be Kept for Future Needs? 1999; Ornl-6952. Oak Ridge National Library. Available from: http://moltensalt.org/references/static/downloads/pdf/ORNL-6952.pdf
Issue
Related topics
Anti-Cancer Therapeutics, Biopharmaceuticals, Drug Development, Drug Manufacturing, Research & Development (R&D), Technology, Therapeutics








