Advancing AOCs to transform the delivery of RNA therapeutics
Posted: 11 December 2023 | Dr Michael Flanagan, Michael Flanagan | No comments yet
In this article, Dr Michael Flanagan discusses how a new class of medicines called antibody oligonucleotide conjugates (AOCs) have the potential to overcome a major challenge encountered with many established RNA-based therapeutics: delivery to tissues outside the liver. Last year, AOCs demonstrated the first-ever successful targeted delivery of RNA into muscle tissue in humans.


One promising new approach that is positioned to transform the RNA field is a new class of drugs called antibody oligonucleotide conjugates (AOCs)”
Recent clinical advances and product approvals have demonstrated significant progress in the development of first-generation RNA therapeutics, for instance with antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs). Researchers have now demonstrated conclusively that messenger RNA (mRNA) can be considered a valid target for correcting genetic mutations at the RNA level that cause a range of genetic diseases, including many rare diseases.1 However, research related to RNA therapeutics has also consistently reinforced limitations that drug developers are steadfastly working to address – primarily that delivery of most RNA therapies has thus far been limited to the liver and unable to target other tissues in the body. However, one promising new approach that is positioned to transform the RNA field is a new class of drugs called antibody oligonucleotide conjugates (AOCs). These are rapidly progressing in clinical development and recently demonstrated the first-ever successful targeted delivery of RNA to muscle tissue in humans.2
Assessing progress in RNA delivery
Techniques in RNA delivery have improved significantly since the introduction of oligonucleotide therapeutics, but there is still much progress to be made. In the modality’s infancy, developers of oligonucleotide therapeutics administered large amounts of synthetic oligonucleotides to ensure effective cellular uptake in the body. Since then, researchers have identified different mechanisms that can allow oligonucleotides to target specific tissues and the root causes of different diseases more effectively. Some therapies are delivered using local injection. The first approved ASO was designed for injection directly into the eye for the local treatment of cytomegalovirus (CMV) retinitis in immunocompromised AIDS patients.3 The drug, fomivirsen, was designed to be complementary to a sequence in CMV mRNAs encoding the major immediate-early region 2 (IE2) proteins, which are essential for CMV replication. Fomivirsen binds to the target mRNA, resulting in inhibition of IE2 protein synthesis and thus inhibition of viral replication, with no systemic absorption observed after intravitreal administration.3,4 There are also numerous drugs in development or approved that use intrathecal administration to target dysfunctional mRNA associated with certain diseases of the central nervous system (CNS).5 Currently, ASOs cannot readily cross the blood-brain barrier and must therefore be delivered intrathecally to ensure direct administration into the CNS for the treatment of neurological diseases.6 Using this delivery method, two ASOs thus far have been approved: one for the treatment of spinal muscular atrophy (SMA) and one for amyotrophic lateral sclerosis (ALS). Several ASOs are in clinical or pre-clinical development, with several others in early discovery stages, for diseases including Huntington’s disease and Alzheimer’s disease.5
Once researchers demonstrated that it was possible to deliver oligonucleotides to hepatocytes, the liver became a popular target”
While intravitreal and intrathecal administration of ASOs has demonstrated success, a significant advance in RNA delivery was the discovery of the N-Acetylgalactosamine (GalNAc) conjugate, which is a small sugar molecule that can attach to siRNAs and bind to specific receptors on hepatocytes (liver cells), allowing for a subcutaneous mode of administration.7 Once researchers demonstrated that it was possible to deliver oligonucleotides to hepatocytes, the liver became a popular target. There are hundreds of diseases that can be addressed by altering the expression of genes in the liver. But what about diseases that require delivery outside of the liver?
The important role of AOCs
AOCs combine the proven technology of monoclonal antibodies (mAbs) with the precision of oligonucleotide therapies. Based on learnings from ASOs and siRNAs they also leverage unique engineering and drug delivery techniques that enable them to target a broader set of cell types, including many that have been previously untreatable with RNA therapeutics.
Research thus far indicates that AOCs also offer levels of flexibility that have not been achievable with existing RNA therapeutics.7 First-generation RNA therapeutics such as ASOs, siRNAs or phosphorodiamidate morpholino oligomers (PMOs) are designed to either reduce RNA or modify its splicing. They utilise Watson-Crick base-pairing rules to target disease-related mRNAs, resulting in degradation of the target mRNA.8 Others modulate RNA function by binding to splice sites on pre‑mRNAs, which results in the skipping of mutation‑containing exons in diseases such as muscular dystrophy.8
In contrast, AOCs are not limited to RNA degradation or splicing modification alone. They can be composed of various types of oligonucleotides, including siRNAs and PMOs that can be engineered to modify RNA function in different ways. This allows researchers to develop oligonucleotides that are tailored to modulate specific disease processes. Mechanisms of these oligonucleotides can range from reducing expression of a disease-related RNA with siRNAs to correcting aberrant processing of RNAs with splice-modifying oligonucleotides.
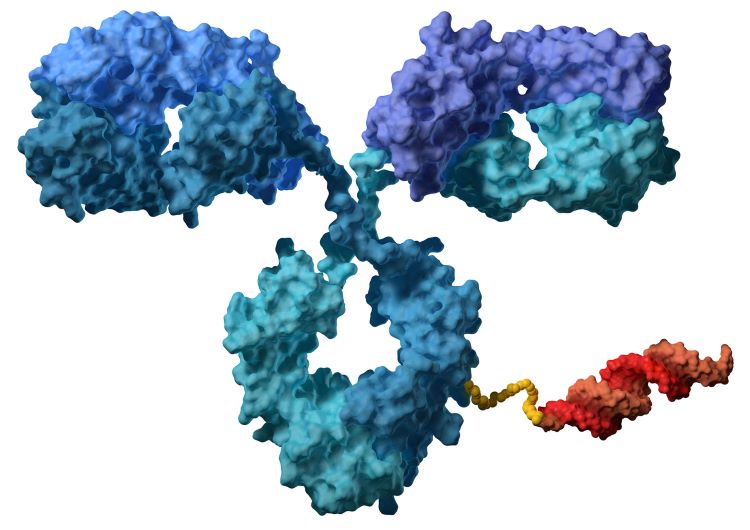

An antibody (blue) linked to an siRNA (red). Credit: Avidity Biosciences
AOCs have three components: mAbs, oligonucleotides and linkers. mAbs have well-established safety profiles, high specificity and affinity and long half-lives – they have been used in drug development for more than 30 years. The oligonucleotides in AOCs are chosen based on factors relating to the target disease and its underlying pathophysiology. Both siRNAs and PMOs are well characterised and have supported the development of drugs with favourable safety profiles, potency in the nanomolar range, and sustained activity in the cytoplasm and nucleus. Known linkers in the RNA field are used to bond together the two pharmacologically active molecular entities and can be applied to multiple oligonucleotide modalities.
Together, these components offer distinct advantages compared to other RNA-targeting approaches, including the flexibility to select and deploy the most potent oligonucleotides, optimal therapeutic durability and the ability to be readily reproducible and scalable. AOCs are synthesised using well‑established and scalable methods for manufacturing mAbs and oligonucleotides. AOCs also use a single mAb across multiple programmes, providing significant leverage around development costs and timelines associated with each programme.
Results indicate that AOCs achieve productive delivery of oligonucleotides to target muscle tissue that has been generally inaccessible to RNA therapeutics”
Most importantly, however; AOCs have demonstrated an ability to target tissue and cell types beyond the liver.7 Recently published pre-clinical data7 showed that AOCs achieved a >15-fold higher concentration in muscle tissue compared to unconjugated siRNA alone in mice, and a single dose resulted in a >75 percent reduction of the target mRNA in both mice and non-human primates. mRNA silencing was also targeted, with minimal to no activity in other major organs. In contrast, oligonucleotides conjugated to control antibodies or cholesterol produced no mRNA reduction or were 10-fold less potent, respectively.7 Results indicate that AOCs achieve productive delivery of oligonucleotides to target muscle tissue that has been generally inaccessible to RNA therapeutics. Researchers also observed that the pharmacokinetic and pharmacodynamic properties of AOCs translated across species from mice to non-human primates.
Progress in targeting skeletal muscle
There is a broad range of muscle diseases, including many rare diseases, where optimal therapeutic activity can only be achieved by directly targeting muscle tissues where the underlying dysfunctional mRNA resides. Before AOCs, researchers attempted to develop oligonucleotide therapies involving complex nanoparticle formulations and conjugation to lipids, peptides or ligands to target muscle and other tissues outside of the liver. However, as seen with lipid nanoparticle (LNP) formulations, while these efforts have been successful in delivering oligonucleotides to the liver, delivery to other organ systems has been negligible.7 Their safety profiles have also shown to be unfavourable for broad applicability as treatment options for many diseases including chronic diseases.7 However, extensive pre-clinical data and initial clinical data of AOCs has demonstrated their potential to overcome the limitations of other therapeutic approaches.2,7 AOCs do not use viruses or nanoparticles for delivery. Instead, they leverage the specificity of mAbs to target tissues of interest, including muscle.
Several AOCs are currently in clinical and pre-clinical development to treat muscle diseases including myotonic dystrophy type 1 (DM1), Duchenne muscular dystrophy (DMD) and facioscapulohumeral muscular dystrophy (FSHD). In Decembemer 2022, researchers at Avidity Biosciences presented data from a Phase I/II clinical trial in DM1 confirming the first-ever successful targeted delivery of RNA to muscle tissue in humans.2 Follow-on topline data from this trial presented at the 75th American Academy of Neurology (AAN) Annual Meeting in April 2023 demonstrated functional improvement in multiple clinical outcome measures, disease modification and a favourable safety and tolerability profile in AOC-treated DM1 patients.9 In addition to treating DM1, these results further highlight the potential for RNA therapeutics to target an even broader range of cell and tissue types in the years ahead. If AOCs can successfully deliver RNA into skeletal muscle, through a unique combination of mAbs conjugated to oligonucleotides, they might be positioned to deliver RNA into other tissues and cells, transforming the future of RNA delivery and offering the possibility to treat the root cause of both rare and common diseases.
This recent research progress is building new levels of interest in AOCs and their potential applications. There are now multiple research and development efforts targeting areas of unmet need including in cardiology and immunology. There is also the potential for AOCs to address some neurological diseases beyond neuromuscular diseases such as DM1 by identifying receptors in neurons amenable to binding to mAbs.
Research advances are also helping us learn more about how RNA is processed, potentially leading to new strategies to alter dysfunctional RNA. As we gain a better understanding of how RNA modulates biological effects, we may uncover new ways to leverage oligonucleotides to modulate gene function in the years ahead and profoundly improve people’s lives.
About the author


References
1. Levin AA. Treating Disease at the RNA Level with Oligonucleotides. NEJM. 2019;380:1.
2. Avidity Announces Positive AOC 1001 Phase 1/2 MARINA™ Data Demonstrating First-Ever Successful Targeted Delivery of RNA to Muscle – Revolutionary Advancement for the Field of RNA Therapeutics. [Internet] Avidity Biosciences, Inc. 2022. [cited 2023July]. Available from: https://aviditybiosciences.investorroom.com/2022-12-14-Avidity-Announces-Positive-AOC-1001-Phase-1-2-MARINA-TM-Data-Demonstrating-First-Ever-Successful-Targeted-Delivery-of-RNA-to-Muscle-Revolutionary-Advancement-for-the-Field-of-RNA-Therapeutics.
3. Bradley CA. First antisense drug is approved with fleeting success. [Internet] Nature Portfolio. 2019. [cited 2023July] Available from: https://www.nature.com/articles/d42859-019-00080-6.
4. Paintsil E, Cheng Y-C. Antiviral agents. Encyclopedia of Microbiology (Third Ed). 2009;223–57.
5. Bennett CF, Krainer AR, Cleveland DW. Antisense Oligonucleotide Therapies for Neurodegenerative Diseases. Annu. Rev. Neurosci. 2019 ;42:385-406.
6. Mazur C, Powers B, Zasadny K, et al. Brain pharmacology of intrathecal antisense oligonucleotides revealed through multimodal imaging. JCI Insight. 2019;4(20).
7. Malecova B, Burke RS, Cochran M, et al. Targeted tissue delivery of RNA therapeutics using antibody–oligonucleotide conjugates (aocs). Nucleic Acids Res. 2023;51(12):5901–10.
8. Levin AA. Targeting Therapeutic Oligonucleotides. NEJM. 2017; 376:86-88.
9. Avidity Biosciences Announces Positive Topline Data from AOC 1001 Phase 1/2 MARINA™ Trial Demonstrating Functional Improvement, Disease Modification and Favorable Safety and Tolerability Profile in People Living with Myotonic Dystrophy Type 1. [Internet] Avidity Biosciences, Inc. 2023. [cited 2023July]. Available from: bit.ly/3qGNiN3.
Issue
Related topics
Biopharmaceuticals, Clinical Development, Clinical Trials, Drug Delivery Systems, Drug Development, Drug Safety, Proteins, Research & Development (R&D), Technology, Therapeutics









