Pharmaceutical industry: 2023 in retrospect
Posted: 29 January 2024 | Dave Elder (JPAG Member and David P Elder Consultancy) | No comments yet
In this article, Dave Elder delves deeper into some ongoing developments in topic areas highlighted in European Pharmaceutical Review (EPR) during 2023.


N-Nitrosamines
By far the biggest issue bedevilling industry during 2023 was the continuing N-nitrosamine contamination saga, which was covered in the third issue of EPR 2023.1 Some five years after the initial NDMA (N-nitrosodimethylamine) contamination issue2 initially affecting valsartan drug substance, then other active pharmaceutical ingredients (APIs), eg, sartans, ranitidine, metformin, etc; the toxic short alkyl chain N-nitrosamine issue appears, if not resolved, then well on the way to resolution. In contrast, NDSRIs (N-Nitrosamine drug substance-related impurities) are still a burgeoning issue with many new NDSRIs being reported on a weekly basis.
During 2023, there were five revisions to the European Medicines Agency’s (EMA’s) Questions and Answers (Q&As) for marketing authorisation holders/applicants on the CHMP Opinion for the Article 5(3) of Regulation (EC) No 726/2004 referral on nitrosamine impurities in human medicinal products.3 This serves to highlight the rapidly changing nature of the problem.
These changes are summarised in Table 1.
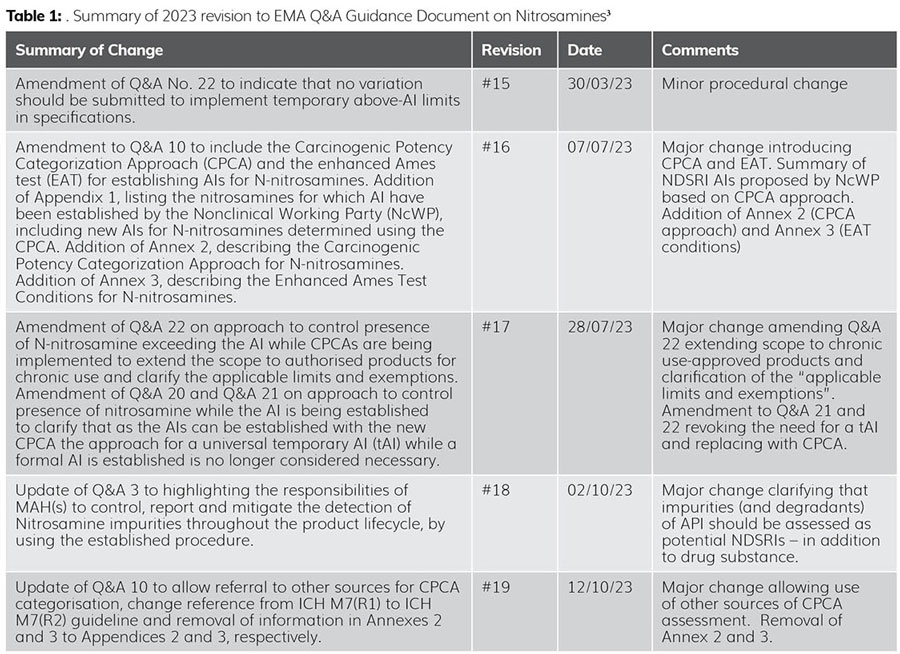

The major take-home message of these ongoing regulatory changes is the realisation that the vast majority of NDSRIs are significantly less toxic than their short alkyl change ‘cousins’, ie, NDMA.4 The use of CPCA assessments allows for the assignment of scientifically justifiable higher acceptable intakes (AIs), without impacting patient safety. Thus, for example, N-nitroso varenicline (NNV) was initially assigned an AI of 37 ng/day even though its molecular size (bridged ring heterocycle) and molecular weight (279.28 g/mol) made it extremely unlikely that NNV would be this toxic, ie, 37 ng/day: that is nearly three times more toxic than NDMA (AI 96 ng/day). Using the CPCA approach, the AI for NNV was re-established at 400 ng/day (ie, over 10X greater) by the EMA, which is aligned with literature values based on structural similarity.5


During 2023, there were five revisions to the European Medicines Agency’s (EMA’s) Questions and Answers (Q&As) for marketing authorisation holders/applicants on the CHMP Opinion for the Article 5(3) of Regulation (EC) No 726/2004 referral on nitrosamine impurities in human medicinal products.3 This serves to highlight the rapidly changing nature of the problem.
In addition, other researchers have claimed that the AIs should be modified to include stoichiometric adjustments, ie, addressing the pronounced differences in molecular weight (MW) between the reference mutagen(s) (typically small MW nitrosamines) and the NDSRI, which have much higher MWs: two-three times greater.6 The CPCA is a pragmatic, science-based approach which ensures that control strategies are more deliverable as method sensitivity/robustness considerations are more easily addressed.
During August 2023 the FDA also updated its guidance for NDSRIs7 and it was closely aligned (but not identical) to EMA’s evolving guidance.3 Differences include that potency category 1 in FDA’s CPCA approach is 26.5 ng/day, compared to EMA which is 18 ng/day. Also, EMA has applied CPCA to over-ride historically assigned AIs, while in some cases FDA has retained those historical limits, eg, NNV8 is still 37 ng/day in US. In these cases, FDA has used structure activity relationships to support the identification of a robustly tested surrogate that is similar in structure and reactivity, ie, read across, to the NDSRI, to generate an estimate of carcinogenic potency from which an AI limit can be scientifically determined. The NNV AI limit is therefore based on the surrogate, N-nitroso-1,2,3,6-tetrahydropyridine (NTHP). FDA has also published the AIs of some 265 NDSRIs.8 Using this list, approximately two-thirds (68 percent) showed strong confidence scores for accurate CPCA potency class predictions. In contrast, only 8 percent showed poor confidence in CPCA potency class predictions and this was typically based on the presence of uncommon structural features that activated or deactivated the molecule.9
Reflecting on five years of quality control for nitrosamine impurities
ICH M7(R2)10 indicates that, “Higher acceptable intakes may be justified when human exposure to the impurity will be much greater from other sources, eg, food or endogenous metabolism (eg, formaldehyde)”. Regulators have struggled with the implications of this guidance as applied to N-nitrosamines given that many foods contain appreciable levels of N-nitrosamines and N-nitrosamines can be endogenously formed from the parent amine and endogenously generated nitrite. Thus, NDSRIs could be generated endogenously.11
In EPR Issue 2 2023,12 the clinical risk-benefit of smoking cessation products was examined. FDA’s AI limit of 37 ng/day for NNV in varenicline (a smoking cessation product) seems increasingly at odds with the huge exposure that patients experience from tobacco-specific nitrosamines (TSNAs); the clear health benefits of stopping smoking and avoiding excessive TSNA exposure seem unfavourably compared with a very constraining AI that has led many manufacturers to exit this market, leading to drug shortages.13
a coherent framework is needed for the derivation of harmonised AIs for novel nitrosamines without robust carcinogenicity data. It is to be hoped that this will be addressed by an update of the ICH M7(R2) guidance during 2024
ICH M7(R2)10 currently provides some guidance to establish AIs for mutagenic impurities. However, AIs for cohort of concern (CoC) mutagenic impurities, eg, N-nitrosamines, are not presently covered. At this current time, the risks posed by N-nitrosamine impurities in medicines have been addressed on a case-by-case basis with some divergence evident between different regulatory authorities, eg, NNV [400 ng/day (EMA) vs 37 ng/day (FDA)]. However, a coherent framework is needed for the derivation of harmonised AIs for novel nitrosamines without robust carcinogenicity data. It is to be hoped that this will be addressed by an update of the ICH M7(R2) guidance during 2024.
Quality risk management
During October 202314 the update to ICH Q9(R1)15 (quality risk management) was discussed. This ICH Q9 revision appears to have been primarily driven by EMA16 who highlighted that “uncertainties and gaps have become apparent during risk assessments for a high number of submissions”. This was particularly apparent in the risk-based decision making arising during the valsartan nitrosamine contamination crisis. It was concluded that an additional focus on “formality, risk-based decision making, subjectivity, and product availability risks”14 was required.
However, the greatest issue with risk assessments still appears to be subjectivity, and the bias inherent in all risk-based decision making. In the valsartan N-nitrosamine contamination issue, the biggest risk was the formation of hydrazoic acid, a byproduct of the sodium azide-mediated cyclisation of the tetrazole ring.16 Hydrazoic acid is a toxic, explosive gas and presents a significant worker-safety risk during manufacture of valsartan drug substance. This, in turn, prompted the use of sodium nitrite as an azide quenching agent, which in turn presents a potential high risk of nitrosamine formation. Risk assessment teams when faced with a confirmed high risk compared to a potential high risk will always prioritise the real risk, especially when the potential risk at that time has not yet been fully explored and nitrosamine exposure from other sources, eg, environment, food, water and endogenous formation, are comparatively high.17
Accelerating drug development
The first issue of 2023,18 dealt with accelerating drug development. A recent publication compared the processes for allowing accelerated marketing authorisations during the COVID‑19 pandemic. The authors highlighted that US and EU regulators were equally effective at accelerating “market access to Covid-19 medicines during the pandemic” without compromising safety or efficacy. However, there were some notable differences. While the EMA’s conditional marketing authorisation only covers first approvals of new treatments, the FDA’s covers “new indications of already-approved treatments”19, ie, repurposing. Repurposing of currently marketed products, eg, methotrexate,20 was an important and effective strategy in combatting COVID-19, particularly in the early stages prior to effective vaccines being developed. During the COVID-19 pandemic (March 2023 – May 2023) the EMA Emergency Task Force (ETF) coordinated and facilitated rapid regulatory activities “for the development, authorisation and safety monitoring of treatments and vaccines intended for the treatment and prevention of COVID-19”.21 These “exceptional measures are no longer applicable, but they may be considered in case of any future public health emergencies”.21 Similar strategies were applied by FDA and other regulatory agencies.22,23
Dissolution
In EPR Issue 4 2023 the “key role that dissolution testing has in the quality control of pharmaceutical drug products, its relevance for poorly soluble compounds, and how its limitations can impact determination of bioavailability”24 was examined. The FDA, in its “Overview of Major Quality Deficiencies and Approaches Available in GDUFA III” (generic drug user fee amendments) identified dissolution testing as one of its major quality deficiencies during 2023. In particular:
- a “new in vitro dissolution (release) analytical method, including development report and validation, is required” as the proposed dissolution method is inadequate
- “data supporting the proposed in vitro release acceptance criteria (eg, in vitro in vivo correlation (IVIVC) data or in silico physiologically based pharmacokinetics (PBPK) modelling) is inadequate”.25
Sun et al.26 assessed FDA field reports between 23005 and 2014 and highlighted that poorly soluble instant release (IR) drug products showed the most dissolution test failures, followed by poorly soluble modified release (MR) drug products and then highly soluble MR drug products.
Lipid-based drug delivery systems (LBDDS)
In EPR Issue 5 2023, lipid-based drug delivery systems (LBDDS) were examined. LBDDS formulations enhance the bioavailability of poorly soluble drugs and “have a proven track record of innovative and commercial success”.27 However, these systems often exhibit poor thermodynamic and/or kinetic stability, which can lead to phase separation on storage.28 In the final quarter of 2023, FDA highlighted that Novartis had issued a voluntary US nationwide recall29 of two batches of Sandimmune® oral solution (Cyclosporine Oral Solution, USP), 100 mg/mL, due to crystallisation. This highlights that even for well established LBDDS formulations, issues arising from thermodynamic instability can still occur.
Conclusions
Of the five major topics covered during 2023, it is likely that all will feature to a greater or lesser extent during 2024. However, N-nitrosamine contamination of drug products will continue to be the principal challenge facing the pharmaceutical industry over this coming year. It is to be hoped that a further revision of ICH M7 will help to address some of the fundamental issues and harmonise regulatory requirements.
References
- Elder DP. Nitrosamines: the beginning of the end? Eur Pharm Rev. 2023; 28(3): 05.
- Schlingemann J, Burns MJ, Ponting DJ, et al. The Landscape of Potential Small and Drug Substance Related Nitrosamines in Pharmaceuticals. J Pharm Sci. 2023; 112: 1287-1304.
- Questions and answers for marketing authorisation holders/applicants on the CHMP Opinion for the Article 5(3) of Regulation (EC) No 726/2004 referral on nitrosamine impurities in human medicinal products. 02 October 2023 EMA/409815/2020 Rev.19.
- Snodin DJ. Mutagenic impurities in pharmaceuticals: A critical assessment of the cohort of concern with a focus on N-nitrosamines. Reg Toxicol Pharmacol. 2023; 141: 105403.
- Dobo KL, Kenyon MO, Dirat O, et al. Practical and Science-Based Strategy for Establishing Acceptable Intakes for Drug Product N-Nitrosamine Impurities. Res. Toxicol. 2022, 35, 3, 475–489.
- Fine J, Allain L, Schlingemann J, et al. Nitrosamine acceptable intakes should consider variation in molecular weight: The implication of stoichiometric DNA damage. Reg Pharm Toxicol. 2023; 145: 105505.
- Recommended Acceptable Intake Limits for Nitrosamine Drug SubstanceRelated Impurities (NDSRIs) FDA Guidance for Industry. August 2023.
- Updated Information | Recommended Acceptable Intake Limits for Nitrosamine Drug Substance-Related Impurities (NDSRIs). December 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/updated-information-recommended-acceptable-intake-limits-nitrosamine-drug-substance-related#compound. Accessed on 02 January 2024.
- Chakravarti S, Saiakhov RD, Girireddy M. Confidence score calculation for the carcinogenic potency categorization approach (CPCA) predictions for N-nitrosamines. Comput Toxicol. 2004; 29: 100298.
- ICH M7(R2). ASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIAL CARCINOGENICRISK. Final version Adopted on 3 April 2023.
- Schmidtsdorff S, Neumann J, Schmidt AH, Parr MK. Risk assessment for nitrosated pharmaceuticals: A future perspective in drug development. Arch Pharm DPhG. 2022; 355: e2100435.
- Elder DP. New quality requirements for tobacco products. Eur Pharm Rev. 2023; 28(2): 05.
- Eckford C. Mitigating medicines shortages in Europe. Eur Pharm Rev. 2023; https://www.europeanpharmaceuticalreview.com/article/188140/mitigating-medicines-shortages-in-europe/. Accessed on 07 January 2024.
- Elder DP. Quality risk management updates. Eur Pharm Rev. 2023; 28(5): 05.
- ICH Q9(R1). Quality risk management.
- Lessons learnt from presence of N-nitrosamine impurities in sartan medicines. EMA/526934/2019. 23 June 2020. https://www.ema.europa.eu/en/documents/report/lessons-learnt-presence-n-nitrosamine-impurities-sartan-medicines_en.pdf. Accessed 14 January 2024.
- Wu Y, Levons J, Narang AS, et al. Reactive impurities in excipients: profiling, identification and mitigation of drug-excipient incompatibility. AAPS PharmSciTech. 2011 Dec;12(4):1248-63.
- Elder DP. Accelerating Drug Development. Eur Pharm Rev. 2023; 28(1): 5.
- Ghadanian M, Schafheutle E. Comparison between European Medicines Agency and US Food and Drug Administration in Granting Accelerated Marketing Authorizations for Covid‑19 Medicines and their Utilized Regulations. Therap Innov & Reg Sci. 2024; 58: 79–113.
- Chen Y-T, Chang Y-H, Pathak N, et al. Methotrexate inhibition of SARS-CoV-2 entry, infection and inflammation revealed by bioinformatics approach and a hamster model. Fron Immunol. 2022: 13; 1080897.
- EMA initiatives for acceleration of development support and evaluation procedures for COVID-19 treatments and vaccines [OBSOLETE]. 2 June 2023 EMA/213341/2020 Rev.5.
- Novack GD. Expedited regulatory product approval in the time of COVID-19. Ocul Surf. 2022 Oct; 26: 345–348.
- FDA Emergency Use Authorization. 12/07/2023. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization. Accessed 17 January 2024.
- Elder DP. Dissolution testing – a dual role. Eur Pharm Rev. 2023; 28(4): 5.
- Ireland K. Overview of Major Quality Deficiencies and Approaches Available in GDUFA III. Generic Drugs Forum 2023 – April 13, 2023.
- Sun D, Hu M, Browing M, et al. Dissolution Failure of Solid Oral Drug Products in Field Alert Reports. J Pharm Sci. 2017; 106(5): 1302-1309.
- Elder DP. Lipid-based drug delivery systems (LBDDS). Eur Pharm Rev. 2023; 28(6): 5.
- Rahman MA, Harwansh R, Mirza MA, et al. Oral lipid-based drug delivery system (LBDDS): formulation, characterization and application: a review. Curr Drug Deliv. 2011; 8(4): 330-345.
- Novartis Issues Voluntary US Nationwide Recall of Two Lots of Sandimmune® Oral Solution (Cyclosporine Oral Solution, USP), 100 mg/mL Due to Crystallization. 11/27/23. https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/novartis-issues-voluntary-us-nationwide-recall-two-lots-sandimmuner-oral-solution-cyclosporine-oral. Accessed 17 January 2024.
Related topics
Dissolution Testing, Drug Development, Regulation & Legislation, Toxicology
Related organisations
European Medicines Agency (EMA), US Food and Drug Administration (FDA)









