Fractionation, purification and downstream processing: the path to commercialisation
Posted: 8 April 2024 | Rob Abbenhuis (Ardena) | No comments yet
With increasing progress in the life sciences, we are now able to treat diseases that were previously deemed incurable. This progress, however, comes with new challenges due to the growing complexity of chemical and biotherapeutic products. Developing and manufacturing compounds of interest in a pure and stable form quickly and efficiently is business critical – if not the decisive factor for success. In this article, Rob Abbenhuis, Chemistry Division Manager at Ardena, explores several key separation and purification techniques.

Credit: Ardena
The growing complexity of chemical synthesis and biotechnological expression systems is associated with a need for more intensive fractionation and purification downstream processes to ensure sufficient product quality. Increasingly stringent quality criteria for purity are required to secure efficacy, potency and stability, and prevent toxicity and immunogenicity, especially for biological products such as proteins, vaccines and monoclonal antibodies. This involves the elimination of any impurities or by-products to achieve the desired compound purity, while maintaining the chemical or biological stability and activity consistently.
The technical challenge is that downstream processes must be specifically tailored for each product, demanding improved understanding to make them more efficient. The more technological options and processes that are available and offered, the more important the expertise in selecting, developing and scaling the most efficient approach from these options becomes.
The goal is to retrieve sufficiently pure compounds required for the development stage, be it an adjuvant or an active compound, to support throughout clinical trials and commercialisation. With downstream processing contributing between 50 and 80 percent to the compound manufacturing costs, increasing the efficiency and simplifying the downstream process has become another important goal for drug development.1
Understanding separation and purification techniques
The upstream processing of chemical synthesis or biotechnology expression systems is a critical step in the manufacture of a specific compound. The process parameters applied determine the levels and types of impurities or by-products present. The upstream process thus determines the complexity and number of fractionation and purification steps required for the downstream process.
Separation and purification of a compound is typically achieved through a variety of different physical and chemical properties which are used to separate the compound of interest from impurities and by-products. These compound-specific properties include size, shape, charge, isoelectric point, charge distribution, hydrophobicity, solubility, density, ligand-binding affinity, metal binding, reversible association, posttranslational modifications and specific sequences or structures.1 The major technologies used include filtration, extraction, precipitation and centrifugation, which are mainly used for harvesting the compound‑containing fraction from the up-scaling. Chromatographic and advanced filtration technologies are used for the final purification step. For complex mixtures, in particular, a scheme of different purification steps is necessary to eliminate impurities and by-products step by step to retrieve the pure compound.
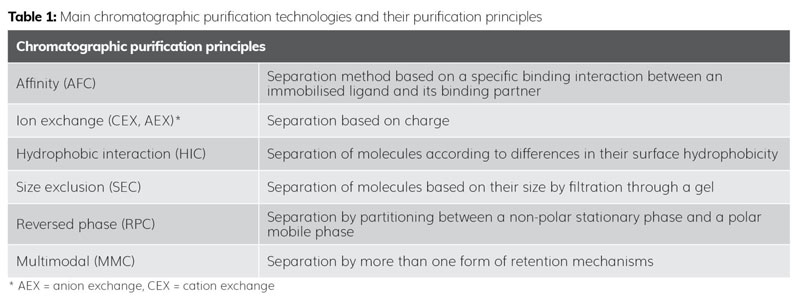

Chromatography is a versatile purification technique that separates the compounds based on their differences in affinity for a particular stationary phase or partitioning between a stationary phase and a mobile phase. This separation is based on the compounds’ physico‑chemical, size or hydrophobic/hydrophilic properties (Table 1). In short, the crude material is applied on a stationary phase (eg, ion exchange resin or functionalised silica), which retains the target compounds to a higher or lower degree than the impurities. The compounds are subsequently separated and eluted by applying an eluent (mobile phase). The purified fractions are collected and water, as well as solvents, can be removed by for example lyophilisation.
Purification by chromatography is a well-understood technology, however, it requires substantial expertise and knowledge as well as selective and sensitive analytical capabilities to successfully evaluate the impact of potentially critical variables.2
Filtration methods generally consist of the following:
- Surface filtration: Use of single-layer filters defined by their pore size
- Depth filtration: Utilising porous matrices, which purify effectively throughout the entire depth and their efficiency capable of being increased through agitation
- Cross-filtration: Technologies such as tangential flow filtration (TFF), which leverage a high-velocity flow tangentially across the membrane surface to purify proteins or nanoparticles
- Diafiltration (DF): A cross-flow filtration technique in which fresh solvent is added to replace the volume loss due to the filtration process
- Ultra-filtration (UF) or nano-filtration (NF): Pressure-driven membrane transport technologies passing mixtures through hollow fibres of membrane material.
Due to the tangential filtration mode, cross-flow filtration technology prevents filter clogging or fouling, which is especially important for the purification of nanoparticles.3 This also applies to the development and commercial manufacturing of new vaccines and vaccine adjuvant nanoparticle products.4
A strategy for effective downstream processing development
A systematic yet practical approach for fractionation and purification is required when starting process development to achieve the desired objectives and milestones in each phase. Table 2 shows some important objectives of fractionation and purification at each stage. It also demonstrates that fractionation and purification should start at the drug discovery phase and continue through to the achievement of a validated and commercially viable downstream process.
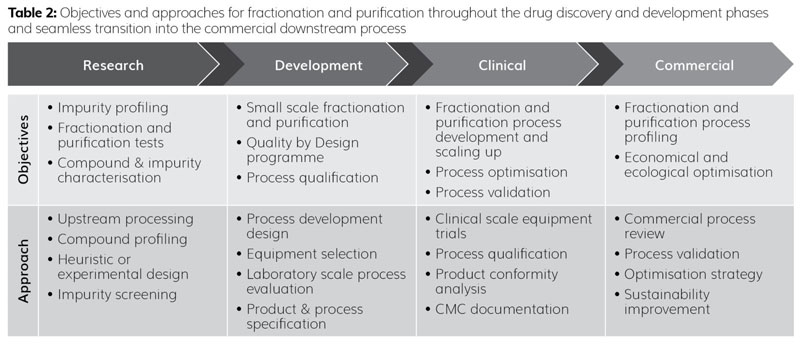

For each compound, whether derived from a biotechnological expression system or natural origin, a rational and product-specific development strategy is required. After reviewing the process and product characteristics, a universal or process-specific heuristic approach can be used effectively for known products and processes.5 In the case of new products and processes, an experimental approach may be more target oriented and can be performed in a high-throughput manner. These high‑throughput process development (HTPD) tools consist of parallel, miniaturised automated systems which can be used for the upstream and downstream processing of product development.6
The need to increase efficiency in separation and purification
In the early phases of a development programme, especially when entering into a Phase I clinical trial, downstream processing is time-critical and the speed of its development is a top priority. However, in the later development phase, the success-critical factors such as the efficiency and scalability of the process come to the fore.
A heuristic approach coupled with HTPD technology based on small-scale automatic dispensing and sampling equipment has proven to be extremely powerful in accelerating downstream development in the early phase while addressing scalability challenges. For example, preparative high‑performance liquid chromatography (HPLC) has evolved as a scalable purification and polishing technology throughout the product lifecycle.7 HTPD technology can also be used to generate large sets of experimental data to support development according to quality-by-design (QbD) principles or a statistical and predictive model-based approach.8 To overcome scalability challenges in later stages along with peptide chromatography purification limitations, catch and release (C&R) methods based on a specific combination of base-labile cleavable linkers with oxime‑based and hydrazone‑based ligation chemistry are under evaluation. However, these methods require more work to further reduce off‑target reactions.9
Enhancing compound concentration and stability can be achieved by eliminating reactive or catalytic impurities through purification, which can be further optimised through an additional lyophilisation process. This may be especially important for overcoming the transport and storage issues associated with vaccines for tropical diseases or use in developing countries, eg, COVID-19 vaccines.10
Purification – a consideration in early-stage drug development
Purification plays an important role at multiple stages of drug development and manufacturing of complex drug synthesis and biotechnology expression systems. Developing an effective and efficient downstream purification process is upstream- and product-dependent and requires individually tailored purification schemes.
It is therefore important to adopt the right approach for the upstream and downstream processes that ensures safe, reproducible purification and can be efficiently transferred to a manufacturing scale. The use of established processes whenever possible can help to avoid complex and lengthy purification procedures and over-engineering.
The challenge of considering commercial manufacturing early in the development phase is often underestimated. The difficulty arises from the wide variety of parameters and equipment variables that are tested during development without consideration for the readiness of the future commercial manufacturing facility.
Science and knowledge-based approaches coupled with strong analytical capabilities accelerate time-critical downstream process development, including the layout towards the most efficient commercial‑scale purification. Depending on their internal infrastructure and organisational setup, companies can greatly benefit from outsourcing development and manufacturing processes.
About the author
Rob Abbenhuis, PhD
After obtaining a PhD in metal-mediated organic synthesis from Utrecht University, Rob started his industrial career in process development and commercialisation of small-molecule APIs. After two years in biotech he worked as Director of Operations in a commercial sterile fill & finish plant. Rob joined Ardena as Director Process R&D & Manufacturing, moving into the role of Business Unit Director of Ardena Oss before his current role. Currently Chemistry Division Manager, Rob is responsible for Ardena’s drug substance activities at sites in Oss (The Netherlands) and Sodertalje (Sweden).
References
- Labrou NE. Protein Purification: An Overview. Methods in Molecular Biology. 2014;1129:3–10.
- Hanke AT, Ottens M. Purifying biopharmaceuticals: knowledge-based chromatographic process development. Trends in Biotechnology. 2014;32(4):210–20.
- Busatto S, Vilanilam G, Ticer T, et al. Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells. 2018;7(12).
- Pires IS, Ni K, Melo MB, et al. Controlled lipid self-assembly for scalable manufacturing of next-generation immune stimulating complexes. Chemical Engineering Journal. 2023;464:142664–4.
- Pfister D, David L, Holzer M, Nicoud RM. Designing affinity chromatographic processes for the capture of antibodies. Part I: A simplified approach. Journal of Chromatography A. 2017;1494:27–39.
- Baumann P, Hubbuch J. Downstream process development strategies for effective bioprocesses: Trends, progress, and combinatorial approaches. Engineering in Life Sciences. 2016;17(11):1142–58.
- De Luca C, Lievore G, Bozza D, et al. Downstream Processing of Therapeutic Peptides by Means of Preparative Liquid Chromatography. Molecules. 2021;26(15):4688.
- Keulen D, Geldhof G, Le Bussy O, et al. Recent advances to accelerate purification process development: A review with a focus on vaccines. Journal of Chromatography A. 2022;1676:463195–5.
- Reimann O, Seitz O, Sarma D, Zitterbart R. A traceless catch-and-release method for rapid peptide purification. Journal of Peptide Science. 2018;25(1):e3136.
- Crommelin DJA, Anchordoquy TJ, Volkin DB, et al Addressing the Cold Reality of mRNA Vaccine Stability. Journal of Pharmaceutical Sciences. 2020;110(3).
Issue
Related topics
Biopharmaceuticals, Bioproduction, Chromatography, Downstream, Drug Manufacturing









