Synchrotron radiation: current status and future landscape
Posted: 24 March 2006 | | No comments yet
Synchrotron radiation (SR) has had a profound impact on the capabilities of structural chemistry and biology. The first dedicated SR X-ray source was the UK’s SRS, which celebrated its 25th Anniversary in 2005. This article provides an overview and several case studies that illustrate the pivotal role that the pharmaceutical industry derives from these tools and methodologies.
Synchrotron radiation (SR) has had a profound impact on the capabilities of structural chemistry and biology. The first dedicated SR X-ray source was the UK’s SRS, which celebrated its 25th Anniversary in 2005. This article provides an overview and several case studies that illustrate the pivotal role that the pharmaceutical industry derives from these tools and methodologies.
Synchrotron radiation (SR) has had a profound impact on the capabilities of structural chemistry and biology. The first dedicated SR X-ray source was the UK’s SRS, which celebrated its 25th Anniversary in 2005. This article provides an overview and several case studies that illustrate the pivotal role that the pharmaceutical industry derives from these tools and methodologies.
An increasing number of large scale SR facilities exist worldwide. The Daresbury Synchrotron Radiation Source (SRS)1 provides state-of-the-art analytical techniques from infrared to hard X-ray wavelengths. The unique characteristics of SR are ideal for analytical problems that require high spatial or temporal resolution or problems that are simply intractable using conventional instruments. The SRS and other large facilities have traditionally been used by universities and higher education institutions for pure research and development. In recognition of the needs of commercial customers, Daresbury Laboratory established DARTS (Daresbury Analytical Research and Technology Service) ten years ago2.
Here we provide practical examples and case histories of how the SRS facilities have been employed in a diverse range of pharmaceutically-relevant research problems. A futuristic vision is then given that takes account of novel X-ray sources, and finally new neutron sources, that are emerging on the horizon and which are predicted to radically alter protein structure determination methods.
Synchrotron protein crystallography: the tool of choice for drug design
A main area of interest for the pharmaceutical industry is determination of protein structure. Since many proteins are intrinsically associated with human health and disease, their structure can be key in developing new pharmaceuticals and drug therapies. The most common technique for obtaining precise information about the arrangement of atoms within a protein is X-ray crystallography (PX). In many cases, conventional laboratory facilities cannot produce data of sufficient quality to resolve the structure of proteins. This is due to their complex characteristics such as low scattering power, relatively poor ordering, limited crystal lifetime and susceptibility to radiation damage. Examples of non-proprietary work carried out at the SRS include the anthrax lethal factor3 and the structure of F1-ATPase4 which was a key contributor to the Nobel Prize for Chemistry in 1997.
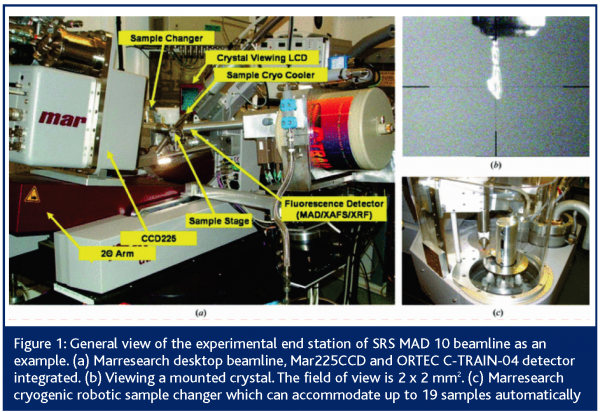
The SRS, like other synchrotrons, has many advantages over traditional laboratory based sources of X-rays: i) The intensity available is typically 1,000 to 10,000 times that emitted by rotating anodes, ii) the SRS X-rays are plane polarised, with emission concentrated in a small forward pointing, flattened cone that delivers most of the photons towards the sample. Rotating anode sources emit spherically and the X-rays go in every direction, leaving a small portion to be delivered to the often tiny sample, size in the range usually of 25 to 500 microns, iii) the SRS X-rays can be selected out of a relatively wide range of wavelengths or energies to optimise the experiment around the sample properties, while the rotating anode can only deliver a fixed wavelength beam of X-rays. As well as tiny crystals very weak scattering can also arise from high solvent content and, especially, a high molecular weight such as with multimacromolecular complexes. These properties also make the SRS capable of performing Multi-wavelength Anomalous Dispersion experiments (MAD), where the radiation wavelength is tuned specifically to exploit the absorption properties of heavier chemical elements that are naturally present in, or deliberately added to, the crystalline sample. The information thus gathered can give vital clues for structure determination. Increasingly, longer wavelengths are being used to make use of the absorption properties of the inherent sulphur content of a protein, thus avoiding the need to add absorbing elements or engineering the protein to include selenium rather than the natural sulphur. Figure 1 shows one such beamline at SRS.
The early years of the SRS from 1980 to 1992 saw the establishment of the first PX instruments (SRS 7.2, SRS 9.6 and SRS 9.5) then in the 1997 to 2004 period a further expansion of PX facilities with SRS MPW 14.1, 14.2 and SRS MPW MAD10. At each phase there was a parallel growth in the community of users and advances in technology (especially detectors), software and methodology. Experiments became possible on time scales that were unthinkable before, such as hours rather than weeks or months and very difficult structural problems were made accessible. The horizon of which projects could be taken on changed radically from 1980 onwards.
Crystallography and structure-based drug design
So what is protein crystallography? Put simply, it is a tool that determines the position of each atom in a molecule in 3D. Why should medicinal discovery take advantage of this tool? Indeed medicines have been used over the generations without intimate knowledge of how they interact with their targets. Pharmaceuticals exert their biological activity by binding to a protein or a gene, usually to suppress its action, but sometimes to enhance it. The chemicals that perform such a biological response were traditionally discovered by accident. Synthetic chemists would then modify parts of the small molecule and test the resultant species, in order to build up a map of activity dependent on the nature and position of new atoms introduced into the compound. This hit-and-miss approach needed two key items of information to optimise the approach: the target of the compound in the system and the method of interaction. While the target can be identified by a host of biochemical methods, the details of the interaction could only be discerned by a 3D structural determination. This is the area where the SRS made an immediate impact.
In the last three decades, crystallography of proteins and other biological molecules started describing such interactions in atomic detail, thus allowing chemists to tailor the design of pharmaceuticals, making them more fit for purpose. The Synchrotron Radiation Source is widely recognised as having made a vital contribution in this effort.
An early commercially important application was the quest for better inhibitors of HIV1 protease, an effort that spanned the globe. Many companies joined the race and there were remarkable successes everywhere5,6, often benefiting from access to the SRS and similar facilities.
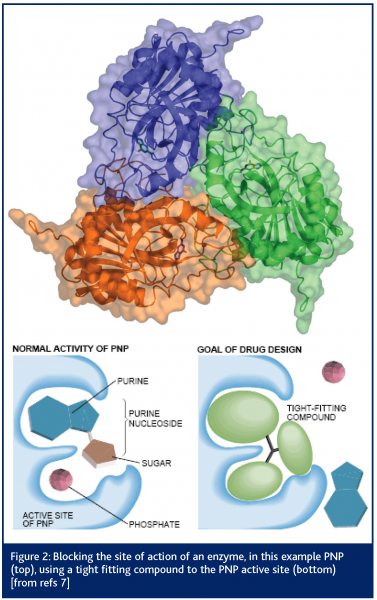
One of the earliest published results from the SRS concerned the structure of the enzyme purine nucleoside phosphorylase (PNP)7. The crystals of this protein contained 80 per cent solvent and yet the protein was a very important drug target (see Figure 2). Diseases such as psoriasis, rheumatoid arthritis, multiple sclerosis and Crohn’s disease appear to have activated T-cells as a major part of their pathogenesis. Therefore, elimination of the presence of such cells could have a profound and beneficial effect on these diseases. Clinical trials with one PNP inhibitor compound, forodesine HCl, support the principle that inhibition of the PNP enzyme has a direct effect on proliferation of activated T-lymphocytes8.
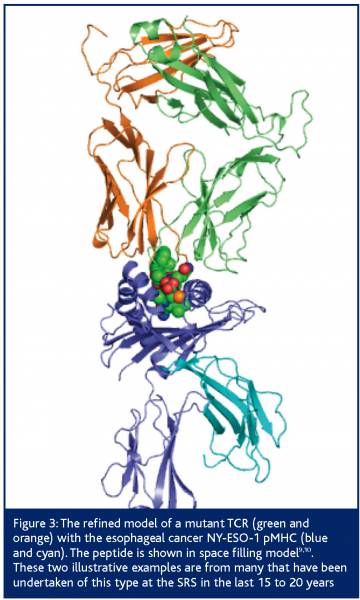
An example of a commercial project conducted by DARTS on behalf of a biotechnology company was the structure determination of wild type and mutant CD8+ T-cell receptors (TCR) and their complexes with cognate Human Leukocyte Antigen-peptide complexes (pMHC); figure 3. TCRs’ affinity is moderated by the thyroid during childhood, to ensure the immune system is not self-selective. As a result, TCR-pMHC affinity is low and chronic or serious diseases and infections are tolerated. Cancer therapy needs antibody like affinity so that the target is carefully selected. The company engineered the TCR that is selective for esophageal cancer to reverse the moderation. Affinity was improved by at least 14,000 fold and the engineered TCR binding concentration was approximately 1 nM or lower9,10.
Figure 3: The refined model of a mutant TCR (green and orange) with the esophageal cancer NY-ESO-1 pMHC (blue and cyan). The peptide is shown in space filling model9,10. These two illustrative examples are from many that have been undertaken of this type at the SRS in the last 15 to 20 years
Synchrotron chemical crystallography
Pharmaceutical companies traditionally take a multidisciplinary approach to their research and hence use a variety of the techniques available on the SRS. A common technique used is single crystal X-ray diffraction for determining the molecular structure of drug molecules. Generally, single crystals can be grown that are large enough to use readily available laboratory-based X-ray instruments. However, in some cases this is not possible and Station 9.8 on the SRS has opened up a new route to obtaining crystal structures. By harnessing the power of the X-rays produced by the synchrotron, researchers can look at increasingly smaller crystals. The smallest crystal currently used for a successful structure determination is 5 x 5 x 5 μm3 11.
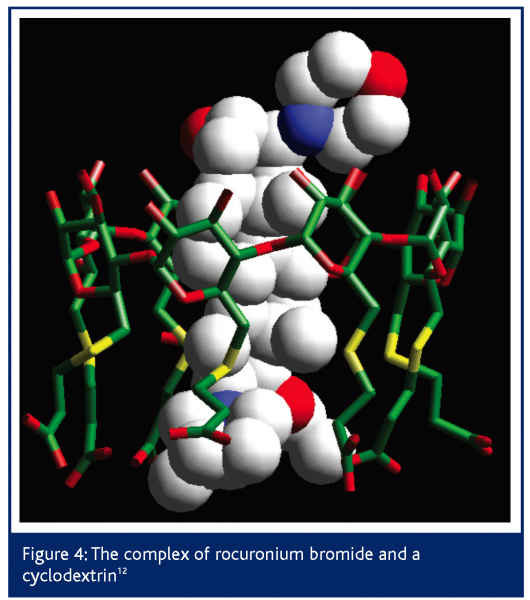
A recent example of a complexed drug structure solved by DARTS was in work for Organon Laboratories12,13. The drug in question is a steroid, rocuronium bromide, which is used as a neuromuscular blocker during surgery under anaesthetic. The action of the drug is reversed by subsequent injection of a cyclodextrin that encapsulates the steroid molecule, thus reversing its blocking effect. Conventional analytical techniques had shown that a complex was formed but not the atomic 3D detail of it.
Due to the complexity of the structure involved and the small size of the crystals, the structure of the complex could not be solved from data obtained using conventional lab X-ray diffractometer equipment because the diffraction was too weak. SRS station 9.8 allowed sufficient data to be collected to solve the structure but, even once the data were collected, the structure solution was less than routine. However, by using the broad expertise at Daresbury, techniques from protein crystallography were used to solve the structure. The structure shown in Figure 4 illustrates how the cyclodextrin binds the rocuronium as predicted. Preliminary trials have shown the cyclodextrin to reverse the effects of the neuromuscular blocker twice as fast as conventional treatments with none of the usual side effects. Human clinical trials are now taking place.
Synchrotron circular dichroism
Where it is not possible to crystallise a particular protein, synchrotron UV circular dichroism (CD) can be used to determine secondary structure content. It has significant advantages over laboratory-based instruments including reduced data collection times, higher signal to noise ratios and extended wavelength range and resolution. CD can also be used as a rapid quality control indicator in protein engineering. The spectra of native and recombinant proteins can be compared quickly to verify correct folding. CD can also be used to study dynamic processes such as protein folding14,15 and misfolding – the basis of diseases such as Alzheimer’s and bovine spongiform encephalitis (BSE). For pharmaceutical companies the opportunity to study drug binding in real time and effects on protein secondary structure offers deeper understanding of potential drug candidates. The SRS is one of only a few facilities worldwide offering this technique and facilities.
Synchrotron powder diffraction
Solution of more complex structures from powder diffraction data is a relatively new technique compared with single crystal structure solution. While it is possible to solve structures from powder diffraction methods using standard laboratory based diffractometers, the low symmetry of many organic materials can make analysis of the patterns troublesome due to severe peak overlap. The fine peak to peak resolution obtainable using the well collimated X-ray beam from the SRS can overcome this problem. Pharmaceutical companies seeking to protect the discovery of a new drug only have adequate protection for their product if their patent covers all of the relevant polymorphic forms. This is also required information in drug approval procedures. In cases of patent infringement DARTS has been instrumental in providing critical evidence that the polymorphic composition of a drug was being copied.
A multi SR source landscape for European researchers
The SR facility and SR research landscape today is very different from when the SRS established itself in the 1980s – and latterly the European Synchrotron Radiation Facility (ESRF) in Grenoble in the late 1990s. Now, at SRS and ESRF, as well as at EMBL and HASY LAB in Hamburg and ELETTRA in Trieste, there are varied SR instruments for protein crystallography, small molecule crystallography and powder diffraction of exceptional technical capability available. There are also new SR facilities under construction in Europe including the Diamond Light Source in the UK, Soleil in France and PETRA 3 in Hamburg which offer new capabilities and capacities.
Ultra-brilliant LINAC X-ray sources being planned include the XFEL (X-ray Free Electron Laser) proposed for construction in Hamburg. This latter new source type is discussed in the context of bringing sample sizes – even of weakly scattering proteins – down to the single molecule range; thus, the bottleneck of protein crystallisation might be sidestepped. Given that a large number of the pharmaceutical industry gene targets are membrane bound, which is a category of protein that is difficult to crystallise, this would be a major step forward for structural science methods.
Furthermore, there is a growth in neutron source and instrument capabilities; even protein crystallography with neutrons is getting around the handicap of needing large protein crystals, a debilitating requirement of the past. Neutrons complement X-rays as a probe, allowing the presence of hydrogen atoms (as deuteriums) to be established at modest crystallographic resolutions like 2.5Å16,17. Many enzyme reactions involve hydrogen and critical residues can be assigned their reacting hydrogen atom, a recent example being aspartic proteinase18. Additionally, all proteins are surrounded naturally by layers of bound waters; neutron diffraction allows a much fuller definition of the interaction with the bound water of an approaching ligand to a protein receptor site. Most recently, fully deuterated aldose reductase has been studied at the Institut Laue Langevin LAue DIffractometer (LADI) with a crystal as small as 0.14 mm3 19. New neutron protein crystallography instruments are being brought to fruition in Grenoble (LADI3) and at the ISIS TS2 (‘LMX++’) near Oxford at the CCLRC Rutherford Appleton Laboratory.
Conclusions
We have illustrated a few examples of the typical industrial problems of relevance to the pharmaceutical industry that have been addressed using the SRS and the DARTS service. The advent of new photon and neutron sources and instruments in Europe and beyond will inevitably lead to increased technical capability for both academic and industrial scientists with the DARTS model for user access ideally suited to the task of managing the activity.




References
- http://www.srs.ac.uk/srs
- http://www.darts.ac.uk
- A.D. Pannifer, T.Y. Wong, R. Schwarzenbacher, M. Renatus, C. Petosa, J. Bienkowska, D.B. Lacy, R.J. Collier, S. Park, S.H. Leppla, P. Hanna, R.C. Liddington, 2001. Crystal structure of the anthrax lethal factor. Nature 414: 229-233.
- J-P. Abrahams, A.G.W. Leslie, R. Lutter, J.E. Walker, 1994. Structure at 2.8-Angstrom resolution of F1-ATPase from bovine heart-mitochondria. Nature 370: 621-628.
- A. Wonacott, R. Cooke, F.R. Hayes, M.M. Hann, H. Jhoti, P. McMeekin, A. Mistry, P. Murray-Rust, O.M. Singh, M.P. Weir, 1993. A series of penicillin-derived C2-symmetrical inhibitors of HIV-1 proteinase – structural and modeling studies. J. Med. Chem. 36: 3113-3119.
- R. Lapatto, T. Blundell, A. Hemmings, J. Overington, A. Wilderspin, S. Wood, J.R. Merson, P.J. Whittle, D.E. Danley, K.F. Geoghegan, S.J. Hawrylik, S.E. Lee, K.G. Scheld, P.M. Hobart,1989. X-ray-analysis of HIV-1 proteinase at 2.7 Å resolution confirms structural homology among retroviral enzymes. Nature 342:299-302.
- C.E. Bugg, W.M. Carson, J.A. Montgomery, 1993. Drugs by design. Scientific American 269: 92-98; Y. S. Babu, S. E. Ealick, C. E. Bugg, M. D. Erion, W. C. Guida, J. A. Montgomery and J. A. Secrist III Structure-based design of inhibitors of purine nucleoside phosphorylase Acta Cryst. (1995). D51, 529-535
- http://www.biocryst.com/bcx_4208.htm
- J.M. Boulter, M. Glick, P.T. Todorov, E. Baston, M. Sami, P. Rizkallah, B.K. Jakobsen, 2003. Stable, soluble T-cell receptor molecules for crystallization and therapeutics. Protein Eng. 16: 707-711.
- J.-L. Chen, G. Stewart-Jones, G. Bossi, N.M. Lissin, L. Woolridge, E.M.L. Choi, G. Held, P.R. Dunbar, R.M. Esnouf, M. Sami, J.M. Boulter, P. Rizkallah, C. Renner, A. Sewell, P.A. van der Merwe, B.K. Jakobsen, G. Griffiths, E.Y. Jones, V. Cerundolo, 2005. Structural and kinetic basis for heightened immunogenicity of T cell vaccines. J. Exp. Med. 201: 1243–1255
- M. Wang, A. Mar, E.J. MacLean, 2003. Structure determination of niobium palladium arsenide, Nb5Pd4As4, from a 5 x 5 x 5 Ìm3 crystal with synchrotron radiation. J. Solid State Chem. 172: 232-236.
- A. Bom, M. Bradley, K. Cameron, J.K. Clark, J. van Egmond, H. Feilden, E.J. MacLean, A.W. Muir, R. Palin, D.C. Rees, M.-Q. Zhang, 2002. A novel concept of reversing neuromuscular block: chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host. Angew. Chemie, Intl. Ed. Engl. 41: 265-270.
- A. Cooper, M. Nutley, E.J. MacLean, K. Cameron, L. Fielding, J. Mestres, R. Palin, 2005. Conformational switches in cyclodextrin-rocuronium complexes. Org. Biomol. Chem. 3: 1863-1871.
- B.A. Wallace, 2000. Synchrotron radiation circular-dichroism spectroscopy as a tool for investigating protein structures. J. Synch. Rad. 7: 289-295.
- D.T. Clarke, G Jones, 2004. CD12: a new high-flux beamline for ultraviolet and vacuum-ultraviolet circular dichroism on the SRS, Daresbury. J. Synchrotron Rad. 11: 142-149.
- M.P. Blakeley, M. Cianci, J.R. Helliwell, P.J. Rizkallah, 2004. Synchrotron and neutron techniques in biological crystallography. Chem. Soc. Rev. 33: 548-557.
- J. Habash, J. Raftery, R. Nuttall, H.J. Price, C. Wilkinson, A.J. Kalb, J.R. Helliwell, 2000. Direct determination of the positions of the deuterium atoms of the bound water in concanavalin A by neutron Laue crystallography. Acta Cryst. D56: 541-550.
- L. Coates, P.T Erskine, S.P. Wood, D.A. Myles, J.B. Cooper, 2001. A neutron Laue diffraction study of endothiapepsin: Implications for the aspartic proteinase mechanism. Biochemistry 40:13149-13157.
- I. Hazemann, M. T. Dauvergne, M. P. Blakeley, F. Meilleur, M. Haertlein, A. Van Dorsselaer, A. Mitschler, D. A. A. Myles and A. Podjarny Acta Cryst. (2005). D61, 1413-1417.




