A Pfizer perspective
Posted: 22 August 2005 | | No comments yet
While the current attention and focus on Process Analytical Technologies (PAT) may make you believe otherwise, PAT measurement systems have been used in the pharmaceutical industry, Pfizer included, for some time, albeit often to a limited extent.
While the current attention and focus on Process Analytical Technologies (PAT) may make you believe otherwise, PAT measurement systems have been used in the pharmaceutical industry, Pfizer included, for some time, albeit often to a limited extent.
While the current attention and focus on Process Analytical Technologies (PAT) may make you believe otherwise, PAT measurement systems have been used in the pharmaceutical industry, Pfizer included, for some time, albeit often to a limited extent.
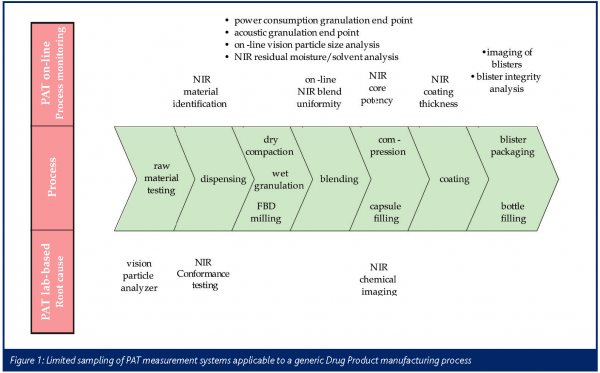
Spectroscopic tools such as UV, IR, NIR and to a lesser extent Raman, as well as less sophisticated measurements like refractive index, temperature, pressure, pH and many more have been employed in Active Pharmaceutical Ingredient (API) process development laboratories for many years to help develop, understand and control reactions, drying processes, crystallizations, etc. Quite a few a these technologies, especially the lower complexity ones, have been carried forward into the API manufacturing areas as essential tools for monitoring and controlling manufacturing processes. On the Drug Product or formulation side, the use of more sophisticated PAT measurement systems may historically not have been at the same level as in the API arena. One of the reasons behind this is the fact that the chemistry in our processes is well understood, but the impact of physical properties is not known to the same extent and therefore it may be more difficult to determine which parameters to measure when developing or manufacturing a formulation. Additionally, there has been a lack of realisation that measurement systems are available or can be adopted or developed to measure just about anything that a formulation scientist or technical services engineer can think of. Figure 1 displays a small sampling of PAT tools that are available for a generic Drug Product manufacturing process.
What has changed?
During the past couple of years, the field of PAT in the pharmaceutical industry has evolved significantly. While there have been many changes (and there are even more still on-going or to come), there are a few that stand out.
First and probably foremost, the US FDA’s “A Drug Quality System for the 21st Century” (formerly known as “GMPs for the 21st Century) initiative as well the creation and publication of the associated PAT guidance document have put an unprecedented focus on PAT as a “system for designing, analyzing, and controlling performance attributes of raw and in-process materials and processes with the goal of ensuring final product quality”. Other regulatory agencies have picked up on this and are developing their own (hopefully harmonised) position on PAT. While according to the regulators PAT has always fitted with Good Manufacturing Practices, the hesitation to implement and utilise such tools based on fear of regulatory unfamiliarity and possible delay of approval or rejection altogether is less of an issue today than it was only a couple of years ago. Having said that, in some areas there is still regulatory uncertainty when it comes to expectations and how to efficiently file these methods. Fortunately, in the US the FDA is actively encouraging and engaging in direct interactions to resolve such issues.
Secondly, a big change in the application of PAT measurement systems as compared to only a couple of years ago is the purpose of their use, especially in the manufacturing area. Previously, PAT tools were most often implemented to address an efficiency issue or quality concern at the single unit operation level. This has evolved to gaining process understanding and control at the process level (from raw materials to API and Drug Product to end product). Additionally these tools are being now used in Root Cause Analysis investigations of manufacturing problems and atypicals. This is certainly the case at Pfizer, as explained in more detail below.
A third significant change is the evolution of PAT vendors and their measurement systems, specifically those focused on pharmaceutical applications. Going back just a few years, the number of vendors capable of delivering systems that could withstand the rigours of a pharmaceutical manufacturing environment and at the same time pass the scrutiny of a quality audit and associated system qualification expectations were far and few in between. Today, you often have a choice of qualified vendors for most common PAT applications (e.g. Near Infrared (NIR) spectrometer systems for raw materials analysis). And then there is the evolution of the technologies themselves and associated data analysis. In many cases PAT measurement systems have become more capable (e.g. higher sensitivity, expanded range/applicability, etc.) and smaller at the same time. Computing power continues to increase, while prices continue to drop; the computing capabilities needed to collect, process, store and analyze large, multidimensional data sets (e.g. spectral imaging data sets) can be found in mid-to-high-end personal computers. More and more vendors are incorporating sophisticated multivariate and statistical data analysis tools into their software.
PAT and Pfizer’s Right First Time Initiative
Several years ago, Pfizer initiated a Right First Time program in the Pfizer Global Manufacturing division. While developed separately, the goal of Right First Time is very well aligned with the FDA Drug Quality and PAT initiatives. According to W. Edwards Deming: “The need for inspection results from excessive variability in the process. The disease is variability…you must have a thorough understanding of the sources of variation in your processes, then work to reduce the variation”. Right First Time is being implemented as the solution to the variability problem. In other words, Right First Time is…
- a deviation reduction process designed to enhance process capability
- a process improvement based on data
- an approach that systematically reveals true root causes
- a means to truly eliminate variability – “it generates consistency and predictability”
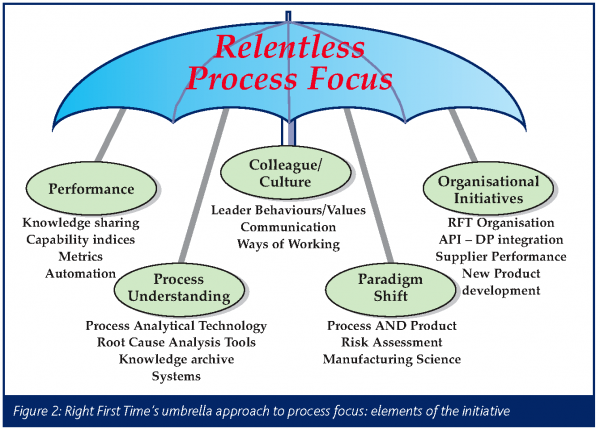
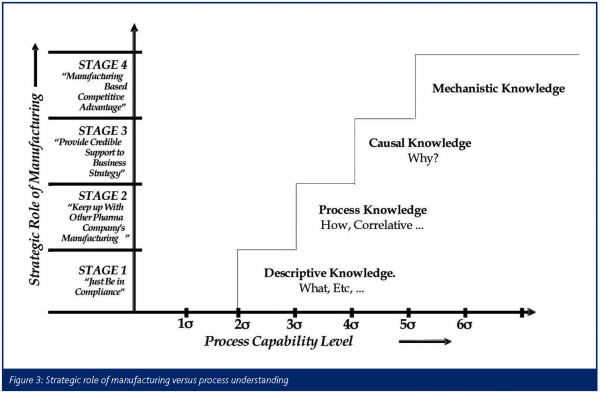
As such, the initiative is focused on the process as shown in Figure 2 which displays its various elements. Once properly implemented, it will allow advancement of the strategic role of manufacturing through descriptive, process, causal, and ultimately mechanistic knowledge of our processes (see Figure 3) with the benefits including, but not being limited to:
- Assurance of product quality
- Enhancement of supply reliability
- Cost improvement
- Inventory reduction
- Cycle time reduction
- Improvement of capacity utilization
- Increase in employee job satisfaction
- Transformation of the organization from reactive through proactive to being predictive
Ultimately, it will allow a transformation from
- End product testing to control of entire processes
- Paper and procedures to process understanding
- A situation in which all change is perceived as ’bad‘ to an environment of continuous improvement
- A setting in which all ’deviations‘ are treated equal to one in which risk assessment is used to evaluate impact on product quality
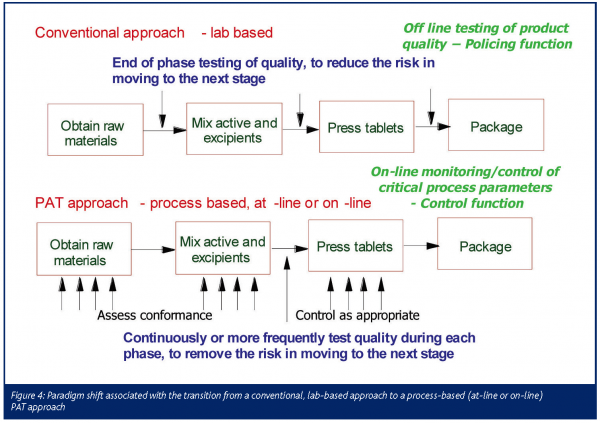
In this whole effort, PAT is only a tool to be applied along with several others as part of a system (Right First Time) to assure manufacturing efficiency and product quality. Specifically, PAT measurement systems are used to monitor the variation throughout the process from raw materials to in-process parameters to finished product attributes. Subsequently, through the use of statistical Design of Experiments and statistical analysis of the data, Critical to Quality attributes and associated Critical Process Parameters are determined. And then ultimately, PAT measurement systems are implemented to remove or control undesired variation in these parameters and attributes. The paradigm shift associated with this approach is graphically displayed in Figure 4. It is important to realise that while multiple PAT measurement systems may be needed to determine Critical to Quality Attributes and Critical Process Parameters, not all of these PAT systems may ultimately be needed to appropriately monitor and control a process.
The advantages of using a PAT process based approach versus a conventional lab based approach are many. First, PAT systems provide information in real-time or near real-time, i.e. in a time frame when one is best able to (re-)act upon it. Most lab-based measurements are not timely enough and thus ’after the fact‘, only allowing verification of quality, but not active and/or predictive control. Additionally, in many cases PAT systems allow continuous or semi-continuous monitoring of a process, providing the ability to detect trends and events that would not be captured by conventional end point detection or (limited) statistical sampling. And with some PAT measurement systems such as for example spectroscopic analyzers, one can collect information on multiple parameters which in turn can be correlated to product performance. Probably most importantly though, PAT puts the information in the hands of the people that can do something with it (the operating staff) which allows quality to be built in as opposed to being tested in.
What still needs to change
As indicated earlier, much has changed during the past few years with respect to PAT. However there are still a number of outstanding issues that need to be addressed to increase the success rate of application and implementation of PAT. The following paragraphs capture some of these, but they are certainly not all-inclusive.
For example, now that PAT has afforded the ability to collect data on large sample sets, specifications applied to ’conventional‘ methods need to be re-evaluated and changed when appropriate. A case in point is the specifications for content uniformity testing: the criteria developed for the harmonised lab-based test with 10 or 30 samples should not be applied to samples sets that are significantly larger (e.g. 100 or more samples). Another specifications-related issue is the question of how to deal with conformance limits which are not necessarily one dimensional, i.e. they typically relate to multiple parameters or characteristics of a raw or in-process material versus conventional specifications which essentially are all one-dimensional.
Another issue that needs attention is the extraction of new information out of old established processes through the use of PAT. In the US, the FDA has created the safe harbor concept, which provides relief from regulatory scrutiny, but only applies during the development phase of the PAT application. Thus the question remains what to do when you find out that your well-established process that you always believed was producing acceptable product (and according to conventional release testing still is…) really is not as stable and/or capable as you thought.
There is also still some uncertainty surrounding the validation of PAT based methods. While the concept of comparability protocols applies (in the US), this assumes a clear direct relationship between the PAT method and the conventional method. But as pointed out before, this relationship may not always be direct and clear, especially when multi-dimensional PAT methods are involved. And in some cases there may very well be no corresponding conventional method because the parameter or attribute was not thought to be of importance and therefore no method and specification was developed. In a similar vein, work still needs to be done in developing an approach for validating PAT-based processes. The abandonment of the conventional 3 batch approach and the introduction of the idea that each batch will be considered a validation batch have certainly provided food for thought, but additional clarity is needed.
Since PAT equipment is most often used on the manufacturing floor, conventional approaches to equipment qualification will need to be adjusted, especially when it comes to on-line equipment performance verification (e.g. the use of traceable standards is often not practical or even possible). And due to the fact that these systems are installed in harsh environments, their ruggedness and reliability is of great importance. The concept of Ruggedness Qualification is now being introduced as an additional component to equipment qualification.
The aforementioned issue of filing mechanisms of PAT methods, especially outside the US requires attention and quick resolution in order not to stifle the PAT and related efforts. Just as a simple illustration: if your company would like to change the release method of a common solvent used in 9 API processes, which end up in 21 formulated products and you market these 21 products in 18 European countries, you need to file 378 variations to update your dossiers in the EU (assuming these products have not been filed under mutual recognition).
Finally, during the recent ’PAT boom‘, it has become clear we are (very) short of qualified personnel to develop and implement these new technologies. Though academia is responding, the number of educational programs in this field is still very limited and often not fully on target. Of course, the issue of education does not only apply to the industry, but the regulators as well.
Fortunately many of the issues mentioned above are being addressed or inroads are being made. Organizations such as the Pharmaceutical Research and Manufacturer’s Association (PhRMA), the Product Quality Research Institute, the European Federation of Pharmaceutical Industries and Associations (EFPIA), the American Society for Testing and Materials (ASTM International) and many more have put together Expert Teams, Committees, etc. to tackle PAT tactical and strategic issues.
In conclusion
Pfizer along with many other pharmaceutical companies, are including PAT in an integrated systems approach to quality assurance and manufacturing efficiency. As such PAT is only one of the many tools being applied to achieve the goal. While the field of PAT has evolved significantly during the past few years, additional work is still required. The good news is that many companies and industry organizations are spending a lot of time and effort to address outstanding issues. And some of these can only be resolved as the industry gains experience: we are at the beginning of a true revolution.