Implementation, validation and registration
Posted: 20 May 2005 | | No comments yet
In the last decade interest in Rapid Microbiological Methods (RMMs) has grown considerably. Technologies such as ATP bioluminescence, solid phase laser cytometry and genetic-based identification systems are being vigorously investigated. Validation and regulatory requirements for such new technologies are beginning to emerge. However, there is a lot of confusion and considerable hesitancy associated with the introduction of these methods into the pharmaceutical sector.
In the last decade interest in Rapid Microbiological Methods (RMMs) has grown considerably. Technologies such as ATP bioluminescence, solid phase laser cytometry and genetic-based identification systems are being vigorously investigated. Validation and regulatory requirements for such new technologies are beginning to emerge. However, there is a lot of confusion and considerable hesitancy associated with the introduction of these methods into the pharmaceutical sector.
In the last decade interest in Rapid Microbiological Methods (RMMs) has grown considerably. Technologies such as ATP bioluminescence, solid phase laser cytometry and genetic-based identification systems are being vigorously investigated. Validation and regulatory requirements for such new technologies are beginning to emerge. However, there is a lot of confusion and considerable hesitancy associated with the introduction of these methods into the pharmaceutical sector.
These new methods rely increasingly on complex technology platforms. The ‘black box’ approach to validation of such technologies is not acceptable. Instrument qualification, computer validation and computer system validation plus microbiological performance testing must all be considered.
An outline of the following areas will be considered in this article:
- Consideration of implementation requirements
- Outline of an integrated validation model for new technology introduction
- Consideration of registration routes for successful implementation
Interest in the area of RMMs is high due to the appearance of a number of RMM test systems designed for use in the pharmaceutical sector. Successful registration and use of RMM test systems is low, however. Uptake of these new methods is still in its infancy, which is strange when you consider similar methods have been successfully adopted by other industrial sectors for several decades. Establishing a clear implementation route will help increase the uptake of these systems and will bring with it many scientific and business benefits for the pharmaceutical industry.
Introducing new microbiological methods into the pharmaceutical sector is no easy task. Broth enrichment, pour plating and use of selective differential agars are the mainstay of modern pharmaceutical microbiology. These conventional methods used for enumerating and identification of microorganisms in pharmaceutical products are far from modern. They have, in fact, been around since the latter part of the nineteenth century. These methods have been highly successful, cheap and simple to perform. Unfortunately they are also labour intensive, take between 5 to 14 days to produce results and require expert interpretation. In addition, they are impossible to automate and will never provide real-time data for accurate and responsive process control. In short, conventional microbiological methods are:
- Potential bottlenecks to production and batch release
- Not suitable for real-time/ near real-time results
Such methods will not provide a suitable platform for future methods. Future methods will need to provide:
- rapid results
- real or near real-time data
- in-line or at-line testing
- automation and high test throughput potential
- highly labour efficient
There is a real need now for the introduction of new methods that can address the requirements of a highly technological industry in the twenty-first century. It is no longer appropriate to use nineteenth century techniques in a modern pharmaceutical industry. The time is right for the uptake of new microbiological methods that will provide solutions for current and future needs.
Implementation
Consider your needs
Before investing in expensive new technology it is important to consider why you need the test system in the first place. This may seem obvious but there are many such ‘technological solutions’ sitting on laboratory benches all across the world doing no more than gathering dust. Some factors to consider in attempting to understand your requirements include:
- what is the purpose of the current test
- do you need to test at all
- what are the real business drivers in changing to new technology
- what are your current/new test requirements
An initial evaluation of any potential new system is highly recommended. A good supplier will offer a loan or short term rental agreement for any prospective customer. Careful consideration needs to be given to this evaluation. The system must be tested for its ability to do the job. It is important that a true impression is gained at this stage. If not a costly mistake with subsequent headaches for unwary users will follow. In addition, it will be all the more difficult in future to persuade managers and financial departments to invest in other new methods.
An important tool in helping persuade management and finance departments of the merits of RMMs is an effective business case. Historically microbiology has not required high capital cost equipment, unlike the more automated chemical analytical laboratory. An effective business case will help enlist the support of senior managers and company finance personnel. Good science alone is not enough in today’s cash strapped industrial environment to secure purchase of a RMM test system.
Some points to consider for a business plan include:
- the true cost of the current test (facilities, reagents, consumables – not just an agar plate!) versus the new system
- labour costs – plus labour savings for the RMM
- effect on batch release
- does the current test reduce operating efficiencies – is it a bottleneck
- quality and compliance issues
- patient benefits
A User Requirement Specification (URS) is a very useful way for the user to define what is required from a new test system. The document is a good foundation for defining what the user wants and what the supplier is being asked to deliver. The URS is the foundation for effective implementation. It also acts as a key reference point for the validation of the system.
PDA Technical Report 33 Vol. 54(3) ‘ Evaluation, Validation & Implementation of New Microbiological Methods’ is an excellent reference document for anyone interested in evaluating RMMs. It gives an overview of vendor implementation, selection, technologies and validation.
Validation
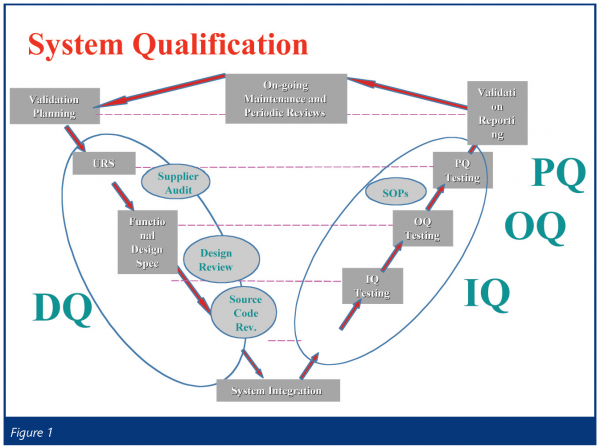
Following a successful initial evaluation and purchase the next step is validation. RMM test systems are increasingly based on complex technological platforms. It is important to focus any validation effort on both the microbiological performance and the technology platform qualification. An effective model for this is the System Qualification Model – used for compute validation and also known as the ‘V’ Model – see Figure 1.
Design Qualification (DQ)
A Validation Master Plan developed at the outset of the validation exercise, which outlines the approach to be taken for the RMM evaluation, is an effective first stage. This will include both equipment and microbiological performance evaluation details.
During DQ user requirements will be outlined and documented in a User Requirement Specification (URS). The document will be used as a basis for defining subsequent validation testing.
Evaluation of computer hardware and software components will be carried out during DQ if considered necessary. A supplier audit may be required due to the novel nature of the application. It may also be required because the system may be bespoke.
Installation Qualification (IQ)
The IQ stage will verify and document that the RMM has been supplied and installed as specified by the supplier.
Operational Qualification (OQ)
OQ will verify and document operating parameters of the RMM by the use of compendial organism and may include environmental organisms. Equipment qualification should be considered at this stage to ensure correct operation of the RMM under working conditions. Individual tests should be outlined in specific protocols with defined acceptance criteria.
Performance Qualification (PQ)
PQ will demonstrate fitness for purpose of the RMM. Testing will comprise of a parallel evaluation of manufactured batches with the registered test method. Testing should be outlined in specific protocols with assigned acceptance criteria. The RMM will be expected to demonstrate equivalence with the existing test method.
Registration
In Europe a new draft regulation ‘European Directive 2001/83/EC’ will give the legal basis for new regulations. Under these new regulations it is proposed that any novel or non-standard technique is likely to be classified as a Major type II variation. Currently such techniques are regarded as Type-I variations. Type-II variations will require:
- an expert report
- formal assessment process
- formal pre-approval letter
This whole process may take 4 to 6 months.
In the United States, the situation is somewhat the reverse. The FDA is actively attempting through a variety of initiatives to simplify or clarify the submission process for RMM technologies which may lead to a simplified reporting route in future.
Introduction of RMM techniques for in-process applications may be more simple. It is important that the user and regulatory authorities have an awareness of the technology/technologies in question if swift implementation is to occur. So it is vital that effective dialogue is established between regulators, users and manufacturers of new test systems to permit swift and painless implementation in the future.
Conclusions
Interest in Rapid Microbiological methods in the pharmaceutical industry is high. There are significant benefits in speed of result, process efficiency savings, sensitivity and business benefits to warrant this interest. Increasingly it is realised that current techniques do not offer a viable platform for future industry requirements in terms of real time or near-real time results and throughput efficiencies.
The advent of PAT has given a new impetus to the introduction of RMMs. Conventional microbiological test methods are not capable of delivering real time or near-real time results, a prerequisite for successful exploitation of PAT benefits. However, rapid methods do have this potential and will be an invaluable aid to successful realisation of PAT objectives.
RMM test systems designed specifically to cater for the requirements of the pharmaceutical industry have been developed in close collaboration between customers and some suppliers. RMM systems are commercially available that are suitable for use in the pharmaceutical sector.
Implementation is a complex topic, however, it is not impossible. Documents and advice are now available that will facilitate successful uptake of RMM test systems. Increasingly dialogue between suppliers, potential users and regulatory authorities is clarifying and defining submission strategies. Registration and use of such systems is happening across Europe and the rest of the world.
Implementation of Rapid Microbiological Methods poses some risks but offers the potential of significant benefits. RMMs also position pharmaceutical microbiology for the future. We must have the courage of our convictions if these benefits are to be realised.

Acknowledgement
This article was originally published in American Pharmaceutical Review, Volume 7, Issue 4, 2004.









