Novel and emerging sterilisation technologies
Posted: 9 October 2009 | | No comments yet
The increasing number and diversity of traditional patient therapies, medical devices, and combinatorial products has created an increasing world-wide interest in the innovation of novel sterilisation technologies. In contrast to traditional moist heat sterilisation processes, emerging technologies are diverse, predominantly reliant on physical processes, involve multiple mechanisms of microbial inactivation and span the complete electromagnetic spectrum. New and emerging technologies include high hydrostatic pressures, pulsed electric fields and low-temperature plasmas that accomplish expedient inactivation of microorganisms at ambient or moderately elevated temperatures. This review presents a high level overview of novel, emerging and developing sterilisation technologies with particular focus upon two potential winners in the race to novel, non-traditional commercially viable modes of sterilisation.
Microbial challenge
All known environments, inanimate and animate surfaces are populated with microorganisms existing as individuals, collectives or consortia. The sheer magnitude of the global microflora can be appreciated by consideration of a single gram of soil in which there exists 108-109 microorganisms. On and within the human body exist 1014 microorganisms, compared to 1013 human eukaryotic cells, therefore in numerical terms we are more microorganism than ‘man’. Microorganisms continually perfect themselves in both morphological and physiological dimensions, by both phenotypic and genotypic mechanisms to fulfill their goal; to endure environmental insult, proliferate, replicate and perpetuate their existence. Microorganisms are capable of replicating at doubling times as small as 20 minutes, and experiencing spontaneous genomic mutation rates close to 1/300 per genome per replication1. Furthermore, recent evidence suggests that mutation rate is actively self regulated under arrested growth rates2, in response to prevailing environmental conditions3 therefore actively self regulating adaptability. These characteristics intrinsic to individuals and microbial populations ensure that there is a continuum of microorganisms physically and physiologically tuned to achieve their goal. The success of such survival strategy is evidenced by the magnitude of microbial species diversity; more than 1.5 x 106 species of microorganisms are believed to exist4. Having existed on earth for two billion years microorganisms have evolved into extremely sensate creatures with the acute capability to measure and monitor their surroundings continuously, instantly and responding accordingly5. Microorganisms routinely communicate to individuals in the immediate vicinity, interacting socially either to individuals of the same species6 or cross-talk to others of different species7. Here their ‘dialogue’ is chemical in nature and akin to pheromone signaling moderating the social interaction of multicellular eukaryotic organisms. There does remain some controversy over the number of microorganisms that are needed to generate sufficient chemical signals to illicit a coordinated social interaction i.e. constituting a ‘quorum’8. This phenomenon of quorum sensing9 is also likely augmented with a microorganism’s inherent capability to sense the environment by diffusion sensing10. Given these facets it would not be inappropriate to regard bacteria as ‘cognitive’ creatures11; exhibiting processes of actively acquiring information, organising sensory inputs to prepensely marshal and coordinate individually or as a social collective. It is only when we consider all these facets of individual microorganisms and collective populations that we perceive the true nature and magnitude of the challenge to sterile and aseptic product manufacture from omnipresent microflora. Therefore, effective, efficient, expedient and economic sterilisation of medical devices and therapies has to be one of the most challenging of manufacturing processes and continues as an opportune area of innovation. The advent of personalised medicine, likely future growth of diverse therapies and compounds (especially in biologics and more ‘fragile’ compounds and formulations) demands an innovative creativity and perhaps an audacious approach to the invention of novel sterilisation technologies.
The birth of sterilisation science
Confrontation between man and microbe has existed for millennia with thermal processes the first recorded and remaining the most widely used form of microbial inactivation. The origins of thermal inactivation can be traced back to Aristotle who advised Alexander’s troops to boil water prior to drinking to sustain their health. Similarly, Hippocrates’s attempted to protect Athenians from plague by igniting controlled fires within the city of Athens. Man’s successful initial adoption of heat to inactivate microorganisms was somewhat borne from serendipity and by no means could be regarded as sterilisation. It was not until the invention of the pressure cooker; attributed to Denis Papin (1647-1712) that an expedient, efficient and consistent means of heat sterilisation was truly established. William Henry in 1831 used heat to disinfect clothing during a cholera outbreak, yet it is Charles Chamberland (1851-1908) who is attributed with initiating research that ultimately led to the invention of the autoclave and therefore should be regarded as the founding father of sterilisation. A few decades later Pasteur, Koch and Wolffhugel all adopted the use of the autoclave (1876-1881). The utility of heat to inactivate microorganisms reflects the multiplicity of macromolecules, cell structures and cellular processes vulnerable to thermal inactivation; thermal inactivation by moist heat involves every cellular component (cell walls, cell membranes, DNA, RNA, enzymes, structural and non-structural proteins12.
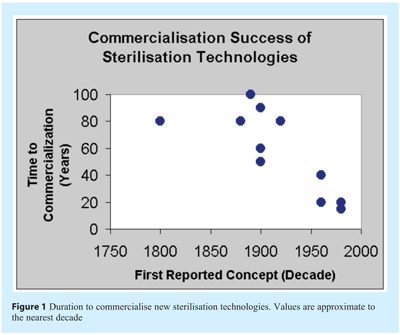
Historically the successful commercialisation of new and alternative sterilisation technologies has been a lengthy process. Currently the duration of time to establish a new sterilisation process with proven commercial capability remains in the region of 10-20 years (see Figure 1).

Defining sterilisation
Before reviewing the status of current, novel and emerging sterilisation technologies it is prudent to examine the definition of the term ‘sterilisation’. A wide variety of definitions are available on the internet, the BusinessDictionary.com describes sterilisation as ‘Total destruction of all microorganisms (whether or not pathogenic) and their spores, usually through the use of drastic methods such as concentrated toxic chemicals (chlorine, formaldehyde, glutaraldehydes, etc.), very high temperature, or intense radiation. A sterilised item cannot support life in any form’. This erroneous definition is especially fascinating as it incorrectly infers that a sterilised item cannot support any form of life. Perusal of other sources of description illustrates the common and erroneous association of the terms ‘destruction’, ‘destroy’ with sterilisation (Answers.com, The FreeDictionary.com,), this perception and the notion that sterilisation processes necessarily are ‘drastic’ and aggressive in nature does not necessarily fit within the design space that modern commercial sterilisation technologies must fit. Biology On Line provides a very succinct definition ‘The act or process of sterilising, or rendering sterile; also, the state of being sterile.’ We must therefore define the state of sterility or being sterile. Chapter <1211> of USP XXXI provides an adequate definition ‘Within the strictest definition of sterility, a specimen would be deemed sterile only when there is complete absence of viable microorganisms from it’. With our current understanding of microbial physiology and epidemiology a more appropriate definition might be ‘The absence of any organism with the potential to manifest a clinical condition’. In truth all microorganisms possess some potential to realise a clinical condition, furthermore this potential to yield harm is not necessarily linked to our capability to recover, culture and enumerate by classic microbiological techniques. Here, our proposed definition permits our inclusion of microorganisms within the broad spectrum of physiological states; including the viable but non-culturable or perhaps more accurately ‘active but non-culturable’ and decouples the traditional link of vitality or viability with sterility. This concept and the significance of culturability, viability, dormancy and clinical implications have been clearly articulated by Kell et al13. The propensity for microorganisms to alter their physiology, conferring varying capabilities to cause infection is not strictly linked to viability, cultivability or even our means of their analytical quantification. The term ‘potential’ therefore allow us to succinctly encompass all conceivable physiological conditions of existence, without having to list these individually.
In the broadest of terms sterilisation technologies render articles sterile (free from any microorganism with the potential to manifest a clinical condition) by three fundamental means:
- Physical Removal – Absolute removal of all microorganisms to render their complete physical absence.
- Physical Alteration – the disintegration or destruction of the microorganism, altering or changing the physical architecture or morphology to remove physiological functionality.
- Inactivation – the permanent disruption of critical inherent biochemical and physiological properties, potential and propensity (whether active or latent) to realise a clinical condition. Ensuring impotency for generating an infection. For complete assurance of inactivation the microorganisms must therefore be essentially ‘killed’ with no residual activity.
Sterilisation: The design space
There exist a number of specific attributes or parameters which form exacting design requirements for any new sterilisation technology. Collectively these requirements describe a design space in which the technology must fit within to satisfy technical, quality, business and regulatory needs (see Figure 2).
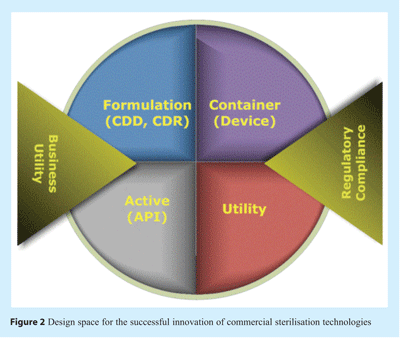

For devices and solution containers the sterilisation technology must be compatible with the material of construction and structure such that geometry, dimension, integrity, continuity of seals, surface and internal formulation chemistries are not adversely altered. Consideration of longer term affects latent immediately after sterilisation but which might develop during shelf-life (especially at higher than room temperature conditions) is necessary. Equally, if the device or container is composed of flexible or pre-designed moveable parts the mechanics of these and how these may affect sterilisation efficacy must be considered.
For therapeutic articles the active agent, solution and formulation must all endure sterilisation without experiencing any alteration in quality, chemistry or efficacy over the duration of shelf-life. Equally any controlled drug delivery system or controlled drug release system must not be detrimentally affected by the process.
Business utility is essential to ensure that the process is expedient, efficient and economically viable. Consideration of the process must also include how sterile items are handled and dispositioned to ensure sustained sterility and release to market; given the fallibility of the sterility test parametric release is preferred and therefore any new mode of sterilisation must carefully consider how future product is released.
These requirements are implicit in regulatory requirements and guidances in that suitability for both product and efficacy of sterilisation must be evidenced. Furthermore, that the demonstration of sterilisation efficacy must be proven using both physical measurements and biological indicators14. For any new sterilisation technology this poses unique challenges. In terms of physical measurement the chemistry and physics of sterilisation processes must be well characterised and understood; based upon pre-established rationale. Yet the expanding portfolio of sterilisation technologies includes increasing numbers of technologies reliant upon multiple mechanisms of microbial inactivation. In contrast the biological indicator of choice may well be unknown. The biological indicator must be sufficiently representative of likely microflora yet should exhibit a greater level of challenge to the sterilisation process (USP XXXI <630>, <862>, <4375>). Moreover the selection of the biological indicator must be clearly and scientifically justified (USP XXXI, 1035). Such difficulty in choice of biological indicator is clearly exemplified in the application of high pressure processing for the sterilisation of articles. Several authors have argued the preference of Bacillus amyloliquafaciens15, Clostridium perfringens16 Geobacillus stearothermophilus17. Ratphitagsanti et al.,18 have summarised the effects of high pressure on the spores of over twenty strains of microorganisms; illustrating that levels of resistance are variable and that choice of a widely recognised test organism remains uncertain. Technological innovations may be widening in spectrum and advancing more swiftly than change in regulatory guidance can currently match; resulting in guidance disparate to state-of-the-art sterilisation science. The FDA recognises this potential and has recommended discussion with the review division to determine appropriateness of the guidance regarding submission filing and content details (FDA Draft Guidance 2008 Parametric Release).
Successful innovation of sterilisation technologies requires an understanding of utility and applicability in the context of intended use. Sterilisation technologies may be applied for four main purposes:
- Terminal sterilisation of final product. Treatment of a complete article within an integral package maintaining sterility after completion of the sterilisation process
- Component or equipment sterilisation. Usually a sterilisation occurring prior to terminal sterilisation, sterile connect, aseptic connect or aseptic manufacture.
- Surface sterilisation. Only surface treatment to render sterile
- Sterile connect. The generation of a completely sterile zone within which sterile components or componentry are simultaneously sterilised, brought together and fitted to generate a sterile unit, maintaining container integrity.
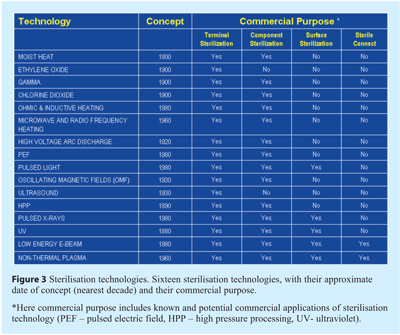
There are approximately two dozen recognised sterilisation technologies existing in various stages of technical and commercial maturity. Figure 3 lists sixteen of these to illustrate the breadth of processes together with (to the authors’ best knowledge) their known or potential commercial application and purpose. The data regarding the first established and recognised concept of each technology is approximated to within a few decades.

Mechanisms of lethality
Four physico-chemical modus operandi for imparting microbial lethality exist forming the fundamental basis of all sterilisation technologies (see Figure 4). Each of these modes have the potential to exert multiple mechanisms which directly alter or adversely affect a microorganism’s morphology, structural integrity or functional integrity resulting in loss of the microorganism’s potential to realise a clinical condition.
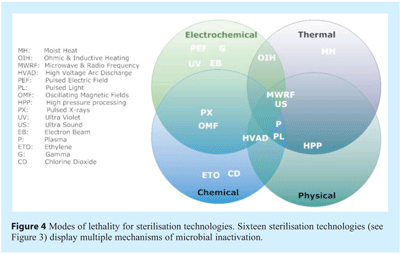

The vast majority of sterilisation technologies exhibit the capability to impart microbial lethality by multiple modes and mechanisms. Ultrasound typifies this, provides a cogent example and deserves further description. Ultrasound, also known as high power ultrasound, ultrasonication or acoustic cavitation imparts microbial lethality via cavitation. The phenomenon of cavitation is broadly defined as the generation, growth and subsequent collapse of cavities resulting in high local energy densities19. Four methods of cavitation generation are recognised; acoustic, hydrodynamic, optic and particle20. Acoustic and hydrodynamic offer the most opportune means of innovative sterilisation technology. The lethal effect of high power ultrasound is due to the physical cavitation phenomena which can occur intracelluarly21 and extracellularly. Cavitation can be classified as stable (or non-inertial) or transient and is dependent upon the energy requirements and operating parameters. Stable cavitation cavities swiftly collapse to a minute fraction of their original size, and the gas dissipates into the environment via a violent mechanism. Concomitantly, extremely high and localised temperatures and pressures are experienced. Such bubbles imploding in an ultrasonic field generate high temperatures reaching 5500oC and pressures exceeding 50,000 KPa22. Heat, pressure shock waves, are all responsible for the lethal effect20 which are manifested in thinning of cell membranes, localised heating and production of free radicals23,24. Mason et al.,25 categorised four mechanisms for ultrasound’s antimicrobial activity, three of which are solely associated with acoustic cavitation:
- Mechanical effects – sheer stresses
- Chemical effects – generation of free radicals
- Heat effects – local hot spots and pressure too.
The fourth was described as a combined treatment effect, experienced with the presence of additional chemicals. Interestingly despite the multiple mechanisms for lethality exacted by ultrasound this has generally proven a poor means of sterilisation with low log reductions (generally <3 log) for vegetative cells and spores (generally <2 log)26. However when used with elevated temperature – thermosonication and increased pressure – manothermosonication there is a modest but significant increase in the delivered lethality27,28.
A common target for imparting lethality
The ubiquitous primary interface between environment and the endogenous biochemical machinery of vegetative bacterial cells (or spores) is the cell envelope; consisting of a cell wall(s) and cell membrane(s). Preservation of both cell wall and cell membrane structural integrity and functionality is fundamental to sustaining the viability and vitality of bacterial cells and spores. Location and the mere fact that these cellular components represent the largest structural and functional continuum make them prime targets for physical and chemical agents to impart lethality29.
Bacterial cell walls are the principal structural scaffold primarily conferring mechanical stability, maintaining cell shape and preventing osmotic lysis. Composed of peptidoglycan, the thickness varies; 10-20 layers in Gram positive bacteria and one to three layers in Gram negative bacteria. In contrast to the vegetative cell wall spores contain a number of biologically-inert layers protecting the inner core and inner membrane. Although bacterial cell and spore walls represent candidate features accessible to physical and chemical damage, whose loss of structural or functional integrity may result in lethality, it has long been known that bacteria can adequately endure and proliferate without cell walls. Such cell wall-deficient bacteria, also termed L-forms are pleomorphic in nature and may occur naturally or can be artificially induced. In comparison to the robust cell and spore walls cell membranes of vegetative cells and spores are more vulnerable to loss of physical and physiological integrity by physical and chemical agents. Gram positive bacteria have a recognised single phospholipids-based cell membrane, Gram negative bacteria have both an outer membrane (lipopolysacharide-based) a phospholipids-based inner membrane, and bacterial spores have an inner and outer membrane. Cell walls satisfy broadly mechanical cellular needs; however cell membranes primarily support and accomplish indispensable physiological processes essential for survival.
Membranes necessarily have to be the conduits of molecules from the environment to the interior of the cell; functioning both as a permeability barrier and a means of modulating trans-membrane transport. Within spores the outer membrane likely plays a less significant role in (im) permeability, whereas the inner membrane may be a major permeability barrier especially to small hydrophilic molecules30. Moir31 has clearly illustrated the pivotal role of the inner membrane of spores in germination and the candidacy of the inner membrane in the loss of heat resistance; either by loss of functional or structural changes. In Gram negative vegetative cells the outer membrane possesses a wide number of porins, receptors, facilitating uptake and efflux of molecules and nutrients32, and are considered to be the first targets of potentially harmful changes that affect membrane integrity and periplasmic function33. The inner membranes are inherently linked to the conservation of free energy by oxidative phosphorylation and provide the means for sustaining viability of all microorganisms.
One seemingly common mechanism of affect on the cell membrane by sterilisation technologies is poration – the generation of transient or sustained transmembrane pores or channels. Figure 5 lists the different types of sterilisation technologies that have been implicated in membrane poration. Since the 1980’s it has been known that the application of electrical fields across cell membranes can swiftly permeabilise the cell membrane through a process that has been termed “electroporation”38. The theory behind electroporation was initially developed by Zimmermann39 and Tsong40 in which an electric field intensity exceeding the critical trans-membrane threshold potential of 1 V across the target cell causes pore formation with an attendant loss of the membrane’s physical and functional integrity. The precise mechanism through which the cell membrane is permeabilised remains uncertain, however it likely involves formation of nano-scale defects or pores in the cell membrane; from which the term “poration” was derived.
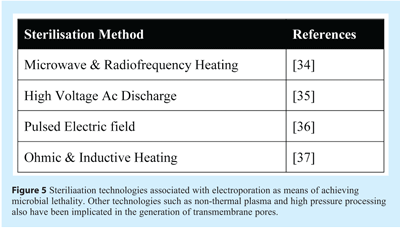

Damage to the physical integrity and architecture of the cell membrane has been demonstrated by transmission and scanning electron microscopy examinations of pulse electric field (PEF) treated bacterial cells41,42. Furthermore evidence that the functional and structural integrity of the membrane is compromised by PEF was provided by Aronsson, Ronner, and Borch43. Here, PEF treated Escherichia coli, Listeria innocua and Saccharomyces cerevisiae increased the uptake of the fluorescent dye propidium iodide (PI) and leakage of intracellular compounds. Some electrical fields generate transitory pores; permeabilising the cell membrane temporarily and permitting the cell’s survival. This process is known as “reversible electroporation” and has become an important and established tool in biotechnology38,44,45,46 and medicine.
Electric fields can be configured to cause the cell membrane to become permanently permeabilised, and result in cell death, by a process referred to as “irreversible electroporation”. Irreversible electroporation may have been observed as early as 1754 when Nollet studied the discharge of a static electrical generator on the skin48. Fuller49 reported that multiple high voltage discharges have bactericidal effect on a water sample. It was the seminal studies of Sale and Hamilton50, Hamilton and Sale51 and Sale and Hamilton52, who demonstrated a non-thermal lethal effect of high electrical fields on organisms in suspensions.
The initial generations of these pores are associated with the increased likelihood of water defects in the membrane interior with increasing applied electric field driven by localised electric field gradients53. Electroporation is exceedingly swift – taking nano-seconds to generate and establish enduring irreversible trans-membrane pores. In practical sterilisation application Katsuki, et al.,54 reported a nanosecond PEF system that studied inactivating vegetative cells of Bacillus subtilis ATCC 6051 suspended in diluted phosphate buffer saline. The field intensity achieved with the nanosecond PEF system was 130 kV/cm, with the pulse width of 45ns, and the pulse rise-time of 2-20ns. The PEF treatment with a shorter pulse rise-time was reportedly more effective in killing the target bacterial cells.
Depending on the particular PEF systems used, typical PEF treatment parameters include pulsed field intensity of 15-50kV/cm, pulse width of 1-5ms, and pulse frequency of 200-400Hz (pulses/s). Most studies in the literature to date, on the use of PEF for microbial inactivation were conducted using systems capable of generating high intensity electric pulses with pulse width in the range of microseconds55. PEF processing parameters included field intensity, pulse width, pulse, and energy input. In addition to the pulse width, pulse shape and polarity are important factors influencing the rate of microbial inactivation. Square-wave pulses are considered to be superior to exponentially decaying pulses as square-waves sustain a constant intensity for the duration of the pulse. Although bipolar pulses have been reported to be more effective for microbial inactivation than monopolar pulses56 several authors57,58 have more recently failed to distinguish a difference upon Gram-positive (Bacillus cereus, Listeria monocytogenes NCTC 11994) or Gram-negative (Escherichia coli NCTC 9001) bacteria.
Although it has been several decades since the first report of microbial inactivation by PEF, the exact mechanisms are yet to be fully understood59. Tieleman53 has modeled the generation of transmembrane pores and has indicated that the membrane curvature accompanies pore formation. That stable, irreversible pore is associated with folding of the membrane lipids with the hydrophilic head groups orientated around the periphery of the pore and along its length (see Figure 6).
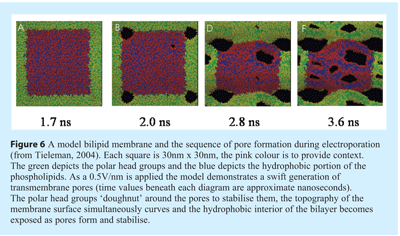

Pulsed electric field (PEF) remains a rapidly developing and emerging technology for microbiological inactivation, with potential as a novel sterilisation technology. In the food industry its first commercial adoption has already been reported for the processing of fruit juices to extend the shelf-life60. However the review of this technology by Wan et al.,55 clearly shows, that despite the potential of PEF log reduction values for vegetative cells, the lack of demonstrated inactivation of spores does not currently support this method as a mature means of sterilisation. A combination of PEF augmented with other sterilisation technologies may well prove an effective means of sterilisation61,55.
Two new and emerging sterilisation technologies are non-thermal plasma-based treatments and high pressure processing, offering wide potential utility (see Figure 3) and deserving of more detailed discussion.
Non-thermal plasma sterilisation
Plasma is commonly regarded as the fourth form of matter, exhibiting properties similar to both gases and liquids, it has been estimated that 99% of our universe is composed of plasma. Plasma is generally a partially ionised gas, with <1% ionised and exhibits a net neutral charge. Application of energy in the form of thermal, electric or magnetic fields and radio or microwave frequencies to a neutral gas results in the generation of plasma. For a review on the generation of non-thermal (low temperature) plasma by electric fields of DC, AC, pulsed DC, radiofrequency, microwave, dielectric barrier, or electron and laser beams62. Electric fields currently remain the most commonly used method of generating plasmas for technological applications.
Low temperature plasmas can be differentiated into atmospheric or low pressure63-65. In atmospheric plasma, the density of gas particles (ions, radicals and molecules) results in a high number of collisions with electrons; leading to rapid exchange of energy between the electrons and heavier particles and resulting in temperatures of the order of 10,000’s degrees Celsius. To maintain a temperature close to room temperature excitation energy can be provided for extremely short periods of time (nanoseconds). A low-temperature plasma can also be generated by maintaining low pressures, here fewer gas particles are present, causing fewer collisions and resulting in high energy electrons and heavier particles with low energy and temperature.
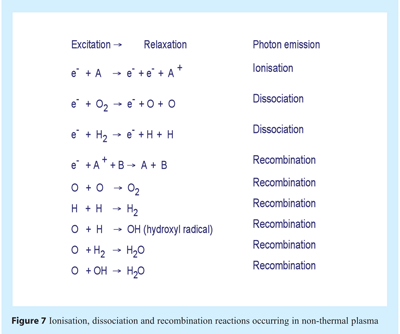
Within plasma smaller charged particles such as electrons move more swiftly than heavier neutral species; with speed and energy increasing until a collision occurs. The collision of electrons with atoms and molecules may result in ionisation or dissociation. An electron may also collide and impart energy to an electron in a low energy shell, the electron ascends to a higher energy shell (excitation) and subsequently returns to the lower energy shell (relaxation). The process of returning to a lower energy shell necessitates release of energy in the form of a photon of light. Collision of electrons with neutral particles caused a cascade of collisions in the gas resulting in the formation of plasma products of electrons, ions, radicals and radiation of varying wavelengths including that in the UV ranges. The plasma attains equilibrium with electrons and charged particles undergoing recombination reactions. Ionisation, dissociation and recombination reactions are illustrated in Figure 7.

Menashi66 first reported upon the sterilising capability of non-thermal plasma; using an RF field to achieve argon plasma at atmospheric pressure and sterilise the inner surface of glass vials. Menashi66 reported the destruction of 106 spores in the vials within one second, however this was artifactual and most likely a product of intense heating (later termed microincineration by Peeples and Anderson67). Over the past two years the potential of non-thermal plasma to inactivate both vegetative and spores has clearly been demonstrated55. These reports demonstrate the utility of this technology and also illustrate that choice of gas, pressure, flow rate, temperature and source of energy as means of plasma generation are critical design parameters. In many of these investigations tri-phasic survivor curves have been determined implying unique mechanisms of inactivation and distinguishing this from classic sterilisation technologies68. It may be argued that there are only two phases, however Moisan et al.,69 interpret the three phases based upon three mechanisms of inactivation with differing kinetics of inactivation:
- UV destruction of genetic material
- UV photon-induced erosion; breaking chemical bonds and leading to the formation of volatile compounds
- Erosion atom by atom, through etching.; the adsorption of reactive species from the plasma subsequently undergo chemical reactions to form volatile compounds
The mechanisms and means of sterilising are highly dependent on the plasma source type and/or the plasma characteristics. For example, gas pressure can profoundly affect the predominant means of inactivation. In low pressure plasmas UV photons likely play a crucial role70,71 at atmospheric pressure, the participation of UV photons remains uncertain, with radicals playing a greater role72,73. Atmospheric pressure plasmas can be generated from a variety of sources, and the variety of plasma characteristics makes it difficult to determine the major mechanism of the microbe’s deactivation. Gweon et al.,74 successfully distinguished oxygen radicals rather than heat, or UV photons as the predominant means of viable cell inactivation.
Application – Non-thermal plasma steriliation has wide application in manufacturing niches in which there is free and ready diffusibility of atoms, molecules and the generation of radicals. Where there is surface to surface contact, solutions, or lyophilised material plasma sterilisation is likely not to be an effective means of sterilisation. Products traditionally sterilised by ethylene oxide are candidates for plasma sterilisation; requiring porous packaging (reference required). Non-thermal plasma also has unrealised potential as providing a sterile field within which sterile containers might be filled or sterile items connected to secure a container maintaining a sterile barrier.
Business utility – In comparison to ionising radiation there are no requirements for shielding. No harmful affects to personnel or the environment. Plasma requires only reasonable investment costs and has low operating costs. Wide range of power requirements; low-temperature sterilisation with plasmas can be realised by running DC discharges, radio-frequency (1-100 MHz) discharges, microwave (<300 MHz) discharges or using very low frequency (1-10 kHz). Most recently Korachi et al.,75 have demonstrated that a low cost atmospheric plasma operating system using either AC or DC power supplies is effective at killing vegetative cells and spores.
Regulatory compliance – Physical measurement for the qualification and validation of plasma sterilisation may be problematic; the mechanism of inactivation is known to vary depending upon the means of plasma generation, therefore a single physical means of measurement is unlikely. If several mechanisms of inactivation are active, then multiple physical means of measurement may be necessary. Currently there is no single and broadly recognised biological indicator. Choice of biological indicator must consider the mechanism of inactivation. Bacterial spores which posses an exosporium may be a prudent choice of candidate indicator organism – adding an extra layer of material to protect against etching.
Products – Plasma has a well recognised potential to affect surface chemistry and alter the physical and chemical properties of plastics and other synthetics. Therefore it may not always be suitable. Alternatively the technology may have a dual benefit including sterilisation and surface treatment. Some products may also be sensitive to the oxidation by electrons and free radicals.
High pressure sterilisation
Although the commercial use of high pressure processing (HPP) is relatively new (currently only used in food processing), the initial idea and proof of concept was achieved first by Hite in 189976. This technology has the unique utility of preserving the organoleptic attributes of many foods during the inactivation of both vegetative cells and spores. As a novel and emerging sterilisation technology this means of microbial inactivation may prove to be efficacious and have negligible adverse affect upon biological molecules or less robust formulations.
In 1914 Hite et al.77 demonstrated that HPP mediated microbial inactivation was not equally effective with all types of foods; likely due to the presence of less susceptible spores on certain items. Patterson78 has reported that spores can be resistant to pressures as high as 1000MPa, and there is growing evidence that HPP must necessarily be augmented with heat to achieve sterilisation79,80.
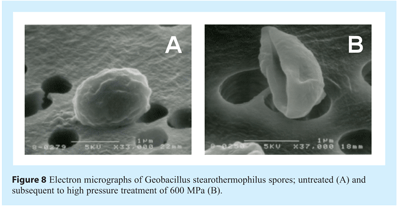
Two distinct mechanisms of spore inactivation have been proposed; each fundamentally dependent upon the magnitude of pressure imposed81,82. Moderately high pressures (50-300 MPa) act upon nutrient-germinant receptors (normally responding to alanine, asparagine, glucose, fructose and potassium), stimulating their activity and triggering the release of Ca2+ and pyridine-2,6-dipicolinic acid (DPA) chelate. There currently remains uncertainty as to if the receptors tertiary or quaternary structures are altered or if the pressure affects the surrounding inner membrane. Release of Ca2+ and DPA initiates a cascade of spore germination events including the initial hydrolysis of spore cortex by cortex-lytic enzymes (CLE), degradation of small acid-soluble proteins (SASPs) and generation of ATP in the similar way to nutrient triggered germination mechanism. The germinated spores are as vulnerable to pressure and heat as vegetative cells. In contrast, high pressures (>500 MPa) induce rapid germination by a mechanical or physical mechanism. Ca2+ DPA is released either via the activation of trans-membrane porins (spanning the spore inner membrane) or by the creation of new channels in the membrane83. Subsequent to Ca2+ and DPA release germination events follow the normal sequence of events. Examination of electron micrographs of spores and flow cytometric analysis of spore’s post-HPP (see Figure 8) suggests that the destructive effect of pressure may be far more catastrophic to spore physical integrity than previously suspected84.

Application – HPP has wide commercial potential in the terminal sterilisation of products or the generation of sterile components. Unlike non-thermal plasma or electron beam this emerging technology is unlikely to present a means of assuring a sterile field for sterile connect applications.
Business utility – The speed of spore inactivation and achievement of multiple spore log reductions is extremely rapid; Khorzad (2008) reporting that thermally assisted HPP achieved a six log reduction of spores within 100 seconds. Careful consideration of this technology is essential to determine the ROI, including the initial cost and subsequent maintenance of equipment which necessarily has to be large and robust to operate under extremes of pressure.
Finale
Further growth in the arsenal of novel sterilisation technologies is certain; driven by our increasing knowledge of microbiology in combination with new and unique products that may not be compatible with traditional sterilisation technologies. Equally it is likely that any new mode of sterilisation will inactivate microorganisms by more than one mechanism and will demand careful partnership with regulators to ensure successful submission approval.
References
- Drake, J.W., Charlesworth, B., Charlesworth D., Crow, J.F. (1998) Rates of spontaneous mutation, Genetics 148 (4), 1667-1686.
- Sniegowski, P. (2004) Evolution: Bacterial mutation in stationary phase. Curr. Biol., 14 (6), R245-R246.
- Massey, R.C., Buckling, A. (2002) Environmental regulation of mutation rates at specific sites. Trends in Microbiol., 10 (12), 580-584.
- Anderson, T-H. (2003) Microbial eco-physiological indicators to asses soil quality. Agriculture Ecosystems and Environment, 98, 285-293.
- Miller, S.I, Hoffman, L.R., Sanowar, S. (2007) Did Bacterial Sensing of host environments evolve from sensing within microbial communities? Cell Host and Microbe, 1(2), 85-87.
- Diggel, S.R., Crusz, S.A., Camara, M. (2007) Quorum sensing. Curr. Biol., 17 (21), R907-R910.
- Henke, J.M., Bassler, B.L. (2004) Bacterial social engagements. Trends in Cell Biol, 14 (11), 648-656.
- Parent, M.E., Snyder, C.E., Kopp, N.D., Velegol, D. (2008) Localized quorum sensing in Vibrio fischeri. Colloids and Surfaces B: Biointerfaces, 62, 180-187.
- Fuqua, W.C., Winans, S.C., Greenberg, E.P. (1994) Quorum sensing in bacteria: LuxR/LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol., 176, 269-275.
- Redfield, R.J. (2002) Is quorum sensing a side effect of diffusion sensing? Trends in Microbiol, 10 (8), 365-370.
- Shapiro, J. A. (2007) Bacteria are small but not stupid: cognition, natural genetic engineering and socio-bacteriology. Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences. 38 (4), 807-819.
- Russell, A.D. (2003a) Lethal effects of heat on bacterial physiology and structure. Sci. Progr., 86, 115-137.
- Kell, D.B., Young, M. (2000) Bacterial dormancy and culturability: the role of autocrine growth factors. Current Opinion in Microbiology, 3(3), 238-243.
- Anon (2008) Annex 1 http://ec.europa.eu/enterprise/ pharmaceuticals/eudralex/vol-4/pdfs-en/2008_02_12_gmp_annex1.pdf
- Rajan, S., Ahn, J., Balasubramaniam, V. M. and Yousef, A. E. (2006). Combined pressure-thermal inactivation kinetics of Bacillus amyloliquefaciens spores in egg patty mince. Journal of Food Protection, 69(4), 853-860
- Paredes-Sabja D., Gonzalez M., Sarker, M.R., Torres, J.A. (2007) Combined Effects of Hydrostatic Pressure, Temperature, and pH on the Inactivation of Spores of Clostridium perfringens Type A and Clostridium sporogenes in Buffer Solutions. J. food Sci., 72(6), M202- M206.
- Furukawa, S. Hayakawa, I. (2000) Investigation of desirable hydrostatic pressure required to sterilize Bacillus stearothermophilus IFO 12550 spores and its sterilization properties in glucose, sodium chloride and ethanol solutions, Food Res. Int. 33(10), 901-905.
- Ratphitagsanti, W., De Lamo-Castellvi, S., Balasubramaniam, V.M. (2009) Bacterial spore inactivation by pressure-assisted thermal processing: challenges in finding suitable biological indicator for process validation. In ‘Biological Indicators For Sterilization Processes’ Gomez, M., Moldenhauer, J. (Eds), DHI publishing, River Grove, IL, USA. 413-450.
- K.S. Suslick, (1990) The chemical effects of ultrasound, Science 247, 1439-1445.
- Gogate, P.R., Kabadi, A.M. (2009) A review of applications of cavitation in biochemical engineering/biotechnology. Biochemical Eng. Journal, 4, 60-72.
- Hughes, D. E., Nyborg, W. L. (1962) Cell disruption by ultrasound. Science, 38:108-114.
- Piyasena, P., Mohareb, E., McKellar, R.C. (2003) Inactivation of microbes using ultrasound: a review. Int J. Food Microbiol, 87, 207-216.
- Butz, P., Tauscher, B.,(2002) Emerging technologies: chemical aspects. Food Research International ,35 (2/3), 279- 284.
- Fellows, P., (2000) Food Processing Technology: Principles and Practice, 2nd ed. CRC Press, New York.
- Mason, T.J., Joyce, E., Phull, S.S., Lorimer, J.P. (2003) Potential uses of ultrasound in the biological decontamination of water, Ultrason. Sonochem. 10, 319-323.
- Anon (2000) Kinetics of Microbial Inactivation for Alternative Food Processing Technologies. A report of the Institute of Food Technologists for the Food and Drug Administration of the U.S. Department of Health and Human Services. http://www.cfsan.fda.gov/~comm/ ift-toc.html.
- Garcia, M. L., Burgos, J., Sanz, B. and Ordonez, J. A. (1989) Effect of heat and ultrasonic waves on the survival of 2 strains of Bacillus subtilis. J Appl Bacteriol. 67:619-628.
- Raso, J., Palop, A., Pagan, R. and Condon, S. (1998). Inactivation of Bacillus subtilis spores by combining ultrasonic waves under pressure and mild heat treatment. J Appl Microbiol. 85, 849-854.
- Russell, A.D. (2003b) Bacterial outer membrane and cell wall penetration and cell destruction by polluting chemical agents and physical conditions. Science Progress, Winter, 1-24.
- Young, S.B. & Setlow, P. (2004) Mechanism of killing of Bacillus subtilis spores by Decon and OxoneTM, two general decontaminants for biological agents. J. Appl. Microbiol., 96, 289-301.
- Moir, A. (2003) Bacterial spore germination and protein mobility. TRENDS in Microbiol., 11 (10), 452-454.
- Hancock, R.E.W. (2001) Pseudomonas aeruginosa outer membrane proteins. http://www.cmdr.ubc.ca/bobh/omps/.
- Behrens, S., Maier, R., de Cock, H., Schmid, F.X. & Gross, C.A. (2001) The SurA periplasmic PpIase lacking its parvulin domains functions in vivo has chaperone activity. The EMBO J., 20, 285-294.
- Kozempel, M. F., Annous, B. A., Cook, R. D., Scullen, O. J. and Whiting, R. C. (1998) Inactivation of microorganisms with microwaves at reduced temperatures. J Food Protect. 61(5), 582-585
- Palaniappan, S., Richter, E. R. and Sastry, S. K. (1990) Effects of electricity on microorganisms: A review. J Food Process Preserv. 14, 393-414.
- Vega-Mercado, H., Pothakamury, U. R., Chang, F.-J., Barbosa-Cánovas, G. V. and Swanson, B. G. (1996). Inactivation of Escherichia coli by combining pH, ionic strength and pulsed electric fields hurdles. Food Res Int. 29(2), 117-121.
- Lee, C. H. and Yoon, S. W. (1999) Effect of ohmic heating on the structure and permeability of the cell membrane of saccharomyces cerevisiae. 1999 IFT Annual Meeting. Chicago. July 24-28.
- Neumann, E., Schaefer-Ridder, M., Wang, Y., Hofschneider, P.H. (2000) Gene transfer intomouse lyoma cells by electroporation in high electrical fields. The EMBO Journal, 1, 841-845.
- Zimmermann, U. (1986). Electrical breakdown, electropermeabilization and electrofusion. Review in Physiology Biochemistry and Pharmacology, 105, 176-257.
- Tsong, T. Y. (1991). Electroporation of cell membranes. Biophysical Journal, 24, 271-295.
- Harrison, S. L., Barbosa-Canovas, G. V., & Swanston, B. G. (1997). Saccharomyces cerevisiae structural changes induced by pulsed electric field treatments. LWT Food Science and Technology, 30(3), 236-240.
- Calderon-Miranda, M. L., Barbosa-Canovas, G. V., & Swanson, B. G. (1999). Transmission electron microscopy of Listeria innocua treated by pulsed electric fields and nisin in skimmed milk. International Journal of Food Microbiology, 51(1), 31-38.
- Aronsson, K., Ronner, U., & Borch, E. (2005). Inactivation of Escherichia coli, Listeria innocua and Saccharomyces cerevisiae in relation to membrane permeabilization and subsequent leakage of intracellular compounds due to pulsed electric field processing. International Journal of Food Microbiology, 99(1), 19-32.
- Zimmermann, U. (1982) Electric field-mediated fusion and related electrical phenomena. Biochimica et Biophysica Acta, 694, 227-277.
- Okino, M., Mohri H. (1987) Effects of a high-voltage electrical impulse andan anticancer drug on in vivo growing tumors. Japanese Journal of Cancer Research, 78, 1319-1321.
- Orlowski, S., Behradek, Jr. J., Paoletti, C., Mir, L. M. (1988) Transient electropermeabilization of cells in culture. Increase in the cyttoxicityof anticancer drugs. Biochem Pharmacol, 37, 4724-4733.
- Rubinsky, B. (2007) Irreversible Electroporation in Medicine. Technology in Cancer Research and Treatment, 6 (4), 255-259.
- Nollet, J. A. Recherches sur les causes particulieres des phénoménes électriques. Paris: Chez H.L. Guerin & L. F. Delatour (1754).
- Fuller, G.W. Report on the investigations into the purification of the Ohio river water at Louisville Kentucky. New York: D. Van Nostrand Company (1898).
- Sale, A. J. H., Hamilton, W. A. (1967) Effects of high electric fields on microorganisms. 1. Killing of bacteria and yeasts. Biochimica etBiophysica Acta, 148, 781-788.
- Hamilton, W. A., Sale, A. J. H. (1967) Effects of high electric fields on microorganisms. 2. Mechanism of action of the lethal effect. Biochimica et Biophysica Acta, 148, 789-800.
- Sale, A. J. H., Hamilton, W. A. (1968) Effects of high electric fields on microorganisms. 3. Lysis of erythrocytes and protopasts. Biochimica et Biophysica Acta, 163, 37-43.
- Tieleman, P. (2004) The molecular basis of electroporation. BMC Biochemistry, 5(10), 1-12.
- Katsuki, S., Moreira, K., Dobbs, E., Joshi, R. P., Schoenbach, K. H. (2002). Bacterial decontamination with nanosecond pulsed electric fields. Proceedings Power Modulator Symposium and High- Voltage Workshop, 648-651.
- Wan, J., Coventry, J., Swiergon, P., Sanguansri P., Versteeg, C. (2009) Advances in innovative processing technologies for microbial inactivation and enhancement of food safety – pulsed electric field and low-temperature plasma. Trends In food Sci. Technol. In press.
- Qin, B. L., Zhang, Q., Barbosa-Canovas, G. V. (1994). Inactivation of microorganisms by pulsed electric fields of different voltage waveforms. IEEE Transactions on Dielectrics and Electrical Insulation, 1, 1047-1057.
- Beveridge, J. R., MacGregor, S. J., Marsili, L., Anderson, J. G., Rowan, N. J., & Farish, O. (2002). Comparison of the effectiveness of biphase and monophase rectangular pulses for the inactivation of microorganisms using pulsed electric fields. IEEE Transactions on Plasma Science, 30(4), 1525-1531.
- Beveridge, J. R., MacGregor, S. J., Anderson, J. G., & Fouracre, R. A. (2005). The influence of pulsed duration on the inactivation of bacteria using monopolar and bipolar profile pulsed electric fields. IEEE Transac ions on Plasma Science, 33(4), 1287-1293.
- Pagan, R., Condon, S., & Raso, J. (2004). Microbial inactivation by pulsed electric fields. In G. V. Barbosa-Canovas, M. S. Tapia, & M. P. Cano (Eds.), Novel food processing technologies, 45-68. CRC Press.
- Clark, J. P. (2006). Pulsed electric field processing. Food Technology, 01.06, 66-67.
- Xin, Q., Zhang, X., Li, Z., Lei, L. (2009) Sterilization of oil-field re-injection water using combination treatment of pulsed electric field and ultrasound. Ultrasonics Sonochemistry, 16(1), 1-3.
- Conrads, H., & Schmidt, M. (2000). Plasma generation and plasma sources. Plasma Sources Science and Technology, 9, 441-454.
- Muranyi, P., Wunderlich, J., & Heise, M. (2007). Sterilization efficiency of a cascaded dielectric barrier discharge. Journal of Applied Microbiology, 103, 1535-1544.
- Laroussi, M. (2005). Low temperature plasma-based sterilization: overview and state-of-art. Plasma Processes and Polymers, 2, 391-400.
- Rossi, F., Kylian, O., & Hasiwa, M. (2006). Decontamination of surfaces by low pressure plasma discharges. Plasma Processes and Polymers, 3, 431-442.
- Menashi, W.P., 1968. Treatment of surfaces. US Patent 3 383 163.
- Peeples, R.E., Anderson, N.R., (1985) Microwave coupled plasma sterilization and depyrogenation I. System characteristics. J. Parent. Sci. Technol. 39, 2-8.
- Hury S., Vidal, D.R., Desor, F., Pelettier, J., Lagarde, T. (1998) A parametric study of the destruction efficiency of Bacillus spores in low pressure oxygen-Based plasmas, Lett. App.l Microbio., Vol. 26, 417-421.
- Moisan, M., Barbeau, J., Moreau , S., Pelletier, J., Tabrizian , M., Yahia, L’H. (2001) Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int. J. Pharmaceutics., 226, 1-21
- Moisan, M., Barbeau, J., Crevier, M.C., Pelletier, J., Philip, N., Saoudi, B., (2002) Plasma sterilization: methods and mechanisms. Pure Appl. Chem. 74, 349-358
- Moreau, S., Moisan, M., Tabrizian, M., Barbeau, J., Pelletier, J., Ricard, A., Yahia, L’H. (2000) Using the flowing afterglow of a plasma to inactivate Bacillus subtilis spores: Influence of the operating conditions. J. Appl. Phys. 88, 1166-1174.
- Deng, X., Shi, J., Kong, M.G. (2006) Physical mechanisms of inactivation of Bacillus subtilis spores using cold atmospheric plasmas. IEEE Trans. Plasma Sci. 34(4), 1310-1316.
- Philip, N., Saoudi, B., Crevier, M., Moisan, M., Barbeau, J., Pelletier, J. (2002) The respective roles of UV photons and oxygen atoms in plasma sterilization at reduced gas pressure: the case of N2-O2 mixtures. IEEE Trans. Plasma Sci. 30 (4), 1429-1436
- Gweon, B., Kim, D.B., Moon S.Y., Choe W. (2008) Escherichia coli deactivation study controlling the atmospheric pressure plasma discharge conditions. Curr. Appl. Phys., 9 (3), 625-628.
- Korachi, M., Turan, Z., Sentu¨rk, K., Sahin, F., Aslan, N. (2009) An investigation into the biocidal effect of high voltage AC/DC atmospheric corona discharges on bacteria, yeasts, fungi and algae. J. Electrosatics, 67(4), 678-685.
- Hite, B.H., (1899) The effect of pressure in the preservation of milk. Bulletin, 58(3), 15-35.
- Hite, B. H., Giddings, N. J. and Weakley Jr., C. E. (1914). The effect of pressure on certain microorganisms encountered in the preservation of fruits and vegetables. West Virginia Agricultural Experiment Station Bulletins, 146, 3-67.
- Patterson, M.F., 2005, Microbiology of pressure-treated foods. J Appl Microbiol, 98(6): 1400-1409.
- Wimalaratne, S.K., Farid, M.M. (2008) Pressure assisted thermal sterilization. Food and Bioproducts Processing, 86, 312-316.
- Wilson, D.R., Dabrowski, L., Stringer, S., Moezelaar, R., Brocklehurst, T.F. (2008) High Pressure in combination with elevated temperature as a method for the sterilisation of food. Trends in Food Science & Technology, 19 (6), 289-299.
- Wuytack, E.Y., Boven S., and Michiels, C. W. (1998). Comparative study of pressure-induced germination of Bacillus subtilis spores at low and high pressures. Applied and Environmental Microbiology, 64(9), 3220-3224.
- Paidhungat, M., Setlow, B., Daniels, W. B., Hoover, D., Papafragkou, E., Setlow, P. (2002). Mechanisms of Induction of Germination of Bacillus subtilis Spores by High Pressure. Appl. Environ. Microbiol., 68(6), 3172-3175.
- Black, E. P., Wei, J., Atluri, S., Cortezzo, D. E., Koziol-Dube, K., Hoover, D. G., Setlow, P. (2007). Analysis of factors influencing the rate of germination of spores of Bacillus subtilis by very high pressure. Journal of Applied Microbiology, 102(1), 65-76.
- Khorzad, A. (2008) PDA 3rd Annual Microbiology Conference. Chicago.







