Specificity in the recombinant factor C test for endotoxin
Posted: 26 April 2018 | Kevin L Williams - bioMerieux | No comments yet
Limulus amebocyte lysate (LAL) users, compendial experts and regulators are still orienting themselves to the recombinant factor C (rFC) assay. Changes to compendial standards do not occur overnight and, for now, users willing to change must perform the alternative validation procedure USP <1225>.


It is this fluorescent sensitivity and specificity that allows rFC to achieve a very sensitive level of detection without the need for the additional cascade proteins: 0.05 EU/mL for a 20-minute test, 0.005 EU/mL for a 60-minute test and 0.001 EU/mL using a 120-minute test.
Level 3. Genetic specificity
It is good to remember where the rFC protein has come from. See Figure 4. The horseshoe crab factor C gene was originally cloned from Carcinoscorpius rotundicauda at the National University of Singapore by Jeak Ling Ding and Bow Ho.9 DNA recombinant technology was developed in the 1970s and culminated with the cloning and expression of the insulin protein as the first recombinant drug (in 1982). The subsequent biotechnology revolution is a powerful story that has culminated in the cloning and expression of dozens of molecules that have drastically improved human health. These drugs include monoclonal antibodies used to treat cancer, infection, and autoimmune disease; cytokines and enzymes used to replace those genetically deficient in specific functions such as blood coagulation. The ‘at will’ expression of natural proteins via recombinant methods can be viewed as perhaps the third great advancement in the prevention and treatment of disease; the first two being increasing sanitation (reducing water-borne illness) and vaccination. Vaccination has come to be improved by DNA recombinant methods, where the antigenic protein can be produced in a purified form thus removing potential risks associated with the use of attenuated organisms (microbial and viral).
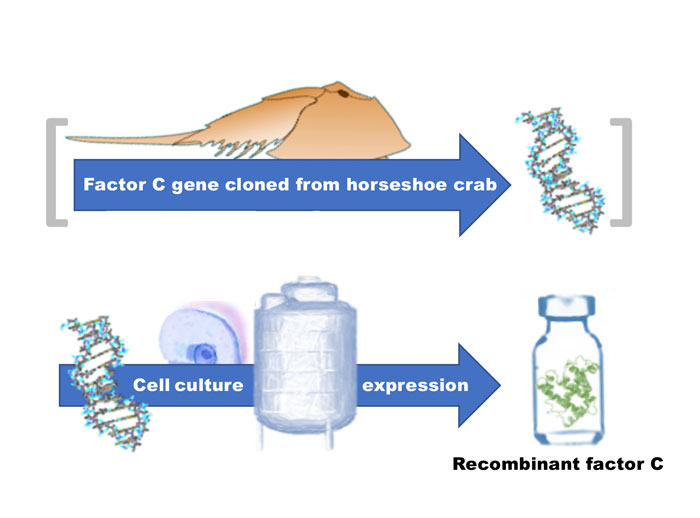

Figure 4: Genetic specificity. Once the gene has been cloned, the desired protein can be produced without the animal from which it was derived, in this case the horseshoe crab biosensor protein factor C
Prior to the availability of recombinant methods some animal proteins, including bovine and porcine insulin, were harvested from cow and pig pancreatic glands beginning in the 1920s. The squeezing of proteins from a mass of organ tissue10 and subsequently processing it for injection requires a great amount of raw materials. According to Diabetes Forecast, more than two tons of pig organs were needed to extract a mere eight ounces of purified animal insulin.11 Biologic drugs today (except for a few vaccines) are produced via biotechnology. Given the anticipated upswing in the number of tests and companies performing LAL testing world-wide, the sustainability of endotoxin via LAL testing is an important concern for the pharmaceutical industry.
The development of animal-based proteins and the subsequent transition to recombinant sourced proteins to protect human health seems an under-appreciated topic. Consider briefly, the insulin story.
In 1921, a young surgeon named Frederick Banting and his assistant Charles Best figured out how to remove insulin from a dog’s pancreas. Skeptical colleagues said the stuff looked like “thick brown muck,” but little did they know this would lead to life and hope for millions of people with diabetes.
With this murky concoction, Banting and Best kept another dog with severe diabetes alive for 70 days – the dog died only when there was no more extract. With this success, the researchers went a step further. A more refined and pure form of insulin was developed, this time from the pancreases of cattle.
In January 1922, Leonard Thompson, a 14-year-old boy dying from diabetes in a Toronto hospital, became the first person to receive an injection of insulin. Within 24 hours, Leonard’s dangerously high blood glucose levels dropped to near-normal levels.
The news about insulin spread around the world like wildfire. In 1923, Banting and Macleod received the Nobel Prize in Medicine.
Soon after, the medical firm Eli Lilly started large-scale production of insulin. It wasn’t long before there was enough insulin to supply the entire North American continent. In the decades to follow, manufacturers developed a variety of slower-acting insulins, the first introduced by Novo Nordisk Pharmaceuticals, Inc., in 1936.12
Of course, the story didn’t end there, as Eli Lilly developed a recombinant version of human insulin which began to be sold in 1982, thus kicking off the recombinant revolution. Lilly has also become one of the first big pharmaceutical companies to pursue testing with rFC in lieu of LAL.13 The ‘at will’ expression of recombinant molecules allows the production of valuable proteins in unlimited amounts in an animal-free manner. The story of recombinant factor C, while not miraculous like the insulin story, provides the similar assurance of a sustainable supply.
References
1. IrFC – An Ixodes ricinus injury-responsive molecule related to Limulus Factor C, Urbanová et al., Developmental & Comparative Immunology, Volume 46, Issue 2, October 2014, Pages 439-447.
2. Definition of endotoxin binding sites in horseshoe crab factor C recombinant sushi proteins and neutralisation of endotoxin by sushi peptides, Tan et al., FASEB J. 2000 Sep;14(12):1801-13.
3. Lipopolysaccharide-sensitive serine-protease zymogen (factor C) of horseshoe crab hemocytes, Identification and alignment of proteolytic fragments produced during the activation show that it is a novel type of serine protease, Tokunaga et al., Eur. J. Biochem. 167,405-416 (1987).
4. Intracellular serine-protease zymogen, factor C, from horseshoe crab hemocytes, Its activation by synthetic lipid A analogues and acidic phospholipids Nakamura et al., Eur. J. Biochem. 176,89-94 (1988) 0 FEBS 1988.
5. The impact of non-endotoxin LAL-reactive materials on Limulus amebocyte lysate analyses, Cooper JF, Weary ME, Jordan FT, PDA J Pharm Sci Technol. 1997 Jan-Feb;51(1):2-6.
6. Sensitivity of Limulus amebocyte lysate (LAL) to LAL-reactive glucans, P F Roslansky and T J Novitsky, J. Clin. Microbiol. November 1991 vol. 29 no. 1.
7. Differential Blocking of Coagulation-Activating Pathways of Limulus Amebocyte Lysate Gui-Hang Zhang et al., Jour. Clin. Microbiology, June 1994, p. 1537-1541.
8. Choosing the Best Detection Method: Absorbance vs. Fluorescence, Jessica Geisler, Posted: May 7, 2015, Biocompare.com, accessed March 21, 2018.
9. US Patent 6,645,724 B1 Nov. 11, 2003.
10. Two tons of pig parts: Making insulin in the 1920s, By Diane Wendt, November 1, 2013, Smithsonian website: O Say Can You See? Stories from the National Museum of American History.
11. Making Insulin, A behind-the-scenes look at producing a lifesaving medication, by Erika Gebel, July 2013 Current Topics, BG Insulin, http://www.diabetesforecast.org/2013/jul/making-insulin.html, Accessed March 16, 2018.
12. The History of a Wonderful Thing We Call Insulin, Posted on August 21, 2012 by American Diabetes Association, http://diabetesstopshere.org/2012/08/21/the-history-of-a-wonderful-thing-we-call-insulin/ accessed March 21, 2018.
13. Application of Recombinant Factor C Reagent for the Detection of Bacterial Endotoxins in Pharmaceutical Products, Bolden J., Smith K.2, PDA J Pharm Sci Technol., 2017 Sep-Oct;71(5):405-412.
Biography


The rest of this content is restricted - login or subscribe free to access


Why subscribe? Join our growing community of thousands of industry professionals and gain access to:
- bi-monthly issues in print and/or digital format
- case studies, whitepapers, webinars and industry-leading content
- breaking news and features
- our extensive online archive of thousands of articles and years of past issues
- ...And it's all free!
Click here to Subscribe today Login here