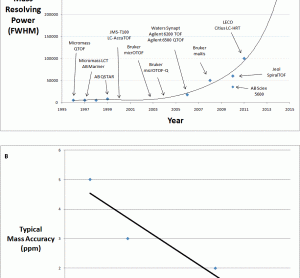
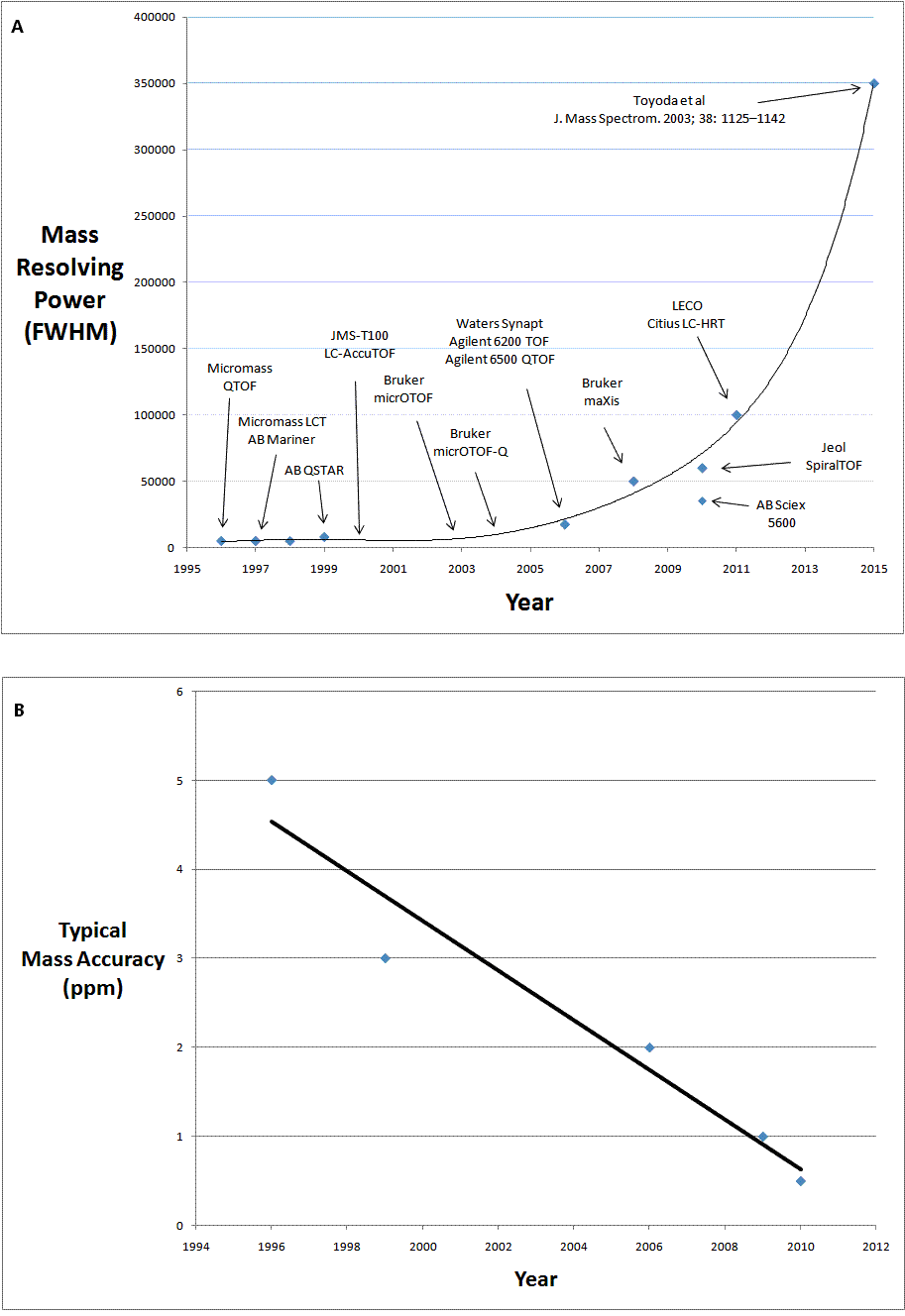
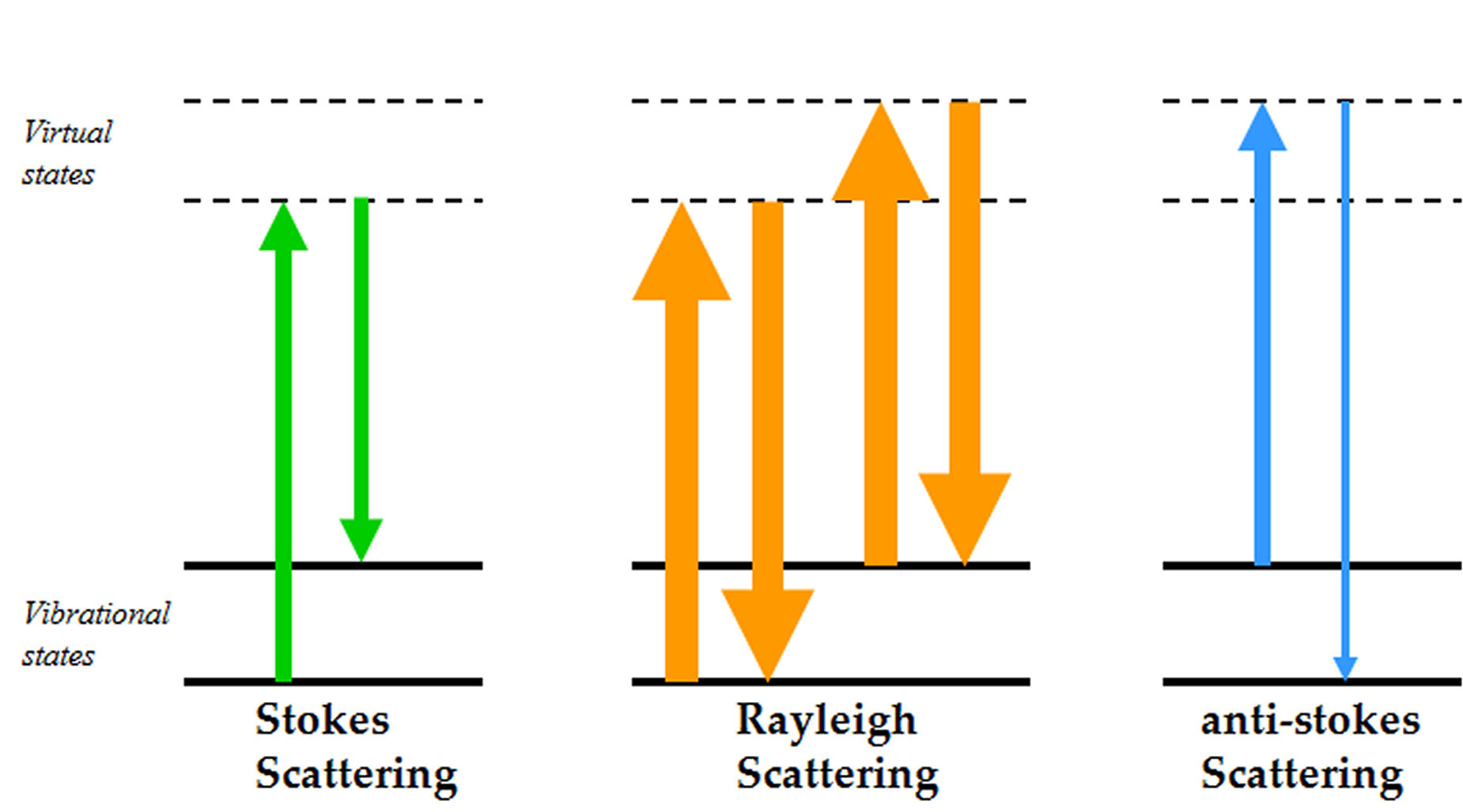
Thermo Fisher Scientific introduces Workflows at AACC 2011
Thermo Fisher Scientific Inc. (NYSE:TMO), the world leader in serving science, today introduced a comprehensive range of clinical research and diagnostic products at the annual conference of the American Association for Clinical Chemistry (AACC). From automated sample handling to innovative sample analysis and storage solutions, ThermoFisher provides an integrated approach…