Analytical technique may advance medical cannabis quality control
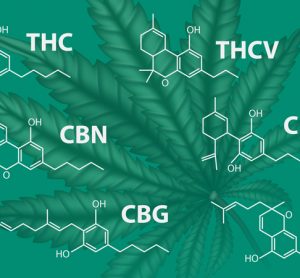
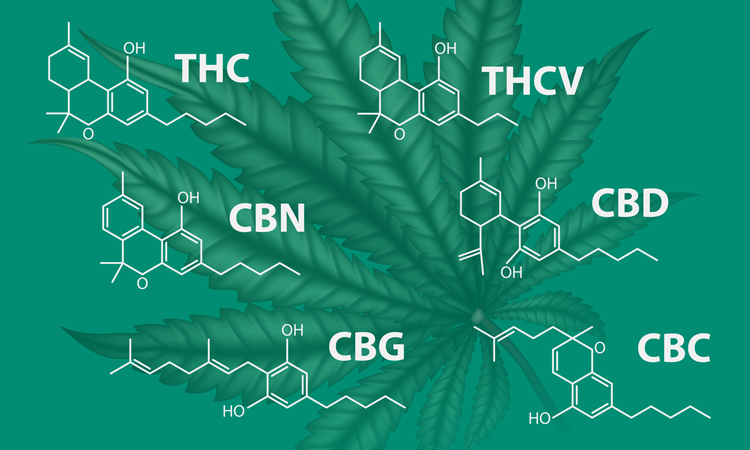
Combining near-infrared spectroscopy (NIR) hyperspectral imaging (HSI) and machine learning could offer a simpler, non-invasive alternative to established analytical techniques such as chromatography, research suggests.