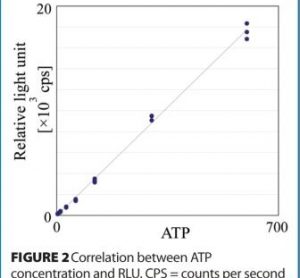
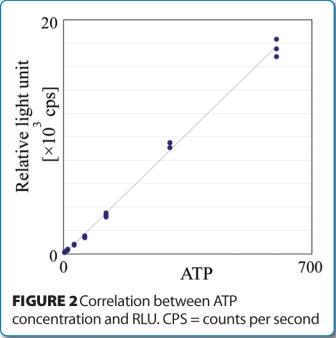
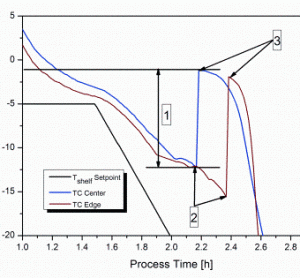
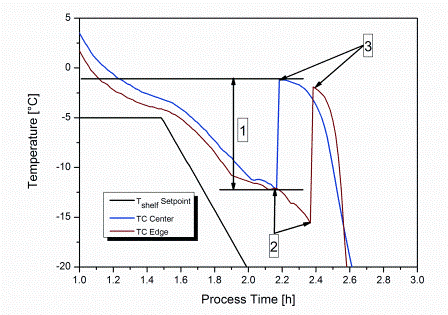
Rapid micro methods and the next generation in ATP bioluminescence
25 October 2012 | By Michael J. Miller, President, Microbiology Consultants, LLC and Noe Miyashita, Researcher, Hitachi Plant Technologies, Ltd
This is the fifth paper in our continuing series on Rapid Microbiological Methods (RMM) that will appear in European Pharmaceutical Review during 2012. As many of you know, I am always on the lookout for the next generation of rapid microbiological method (RMM) technologies and solutions. In this article, I…