FDA approves Jentadueto™ (linagliptin/metformin hydrochloride) tablets for the treatment of adult patients with type 2 diabetes
Posted: 30 January 2012 | | No comments yet
Boehringer Ingelheim Pharmaceuticals, Inc. and Eli Lilly and Company (NYSE: LLY) today announced the U.S. Food and Drug Administration (FDA) has approved Jentadueto™ (linagliptin/metformin hydrochloride) tablets, a new tablet combining the dipeptidyl peptidase-4 (DPP-4) inhibitor, linagliptin, and metformin. JENTADUETO provides a new, single-tablet treatment option, taken twice-daily, for patients who need to control their blood sugar. Linagliptin (5 mg, once-daily) is marketed in the U.S. as Tradjenta™ (linagliptin) tablets.
JENTADUETO is a prescription medication used along with diet and exercise to improve glycemic control in adults with type 2 diabetes when treatment with both linagliptin and metformin is appropriate. At the maximum dose, JENTADUETO demonstrated placebo-corrected reductions in hemoglobin A(1c) (HbA(1c) or A1C) levels of up to 1.7 percent (+0.1 percent for placebo and -1.6 percent for JENTADUETO). A1C is measured in people with diabetes to provide an index of blood sugar control for the previous two to three months. JENTADUETO did not cause any meaningful change in body weight. JENTADUETO can be used alone or in combination with a sulfonylurea, a commonly prescribed medication for type 2 diabetes. JENTADUETO is not for the treatment of type 1 diabetes or diabetic ketoacidosis (increased ketones in the blood or urine). It has not been studied in combination with insulin. The JENTADUETO label contains a boxed warning for the risk of lactic acidosis, a serious metabolic complication that can occur due to metformin accumulation during treatment with JENTADUETO.
“Most people with type 2 diabetes require more than one medication to help lower their blood sugar, due to the complex nature of type 2 diabetes,” said Lance Sloan, MD, Texas Institute for Kidney and Endocrine Disorders. “The approval of JENTADUETO is exciting because it combines two diabetes medications in a single tablet, making it a good option for people who need an additional medication, and for whom both linagliptin and metformin is appropriate.”
JENTADUETO Clinical Trials
In a 24-week, randomized, double-blind, placebo-controlled factorial study evaluating 791 adult patients with type 2 diabetes and inadequate glycemic control with diet and exercise, linagliptin plus metformin demonstrated the following:
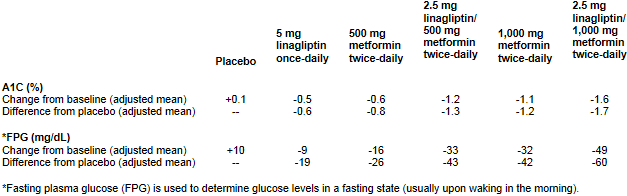
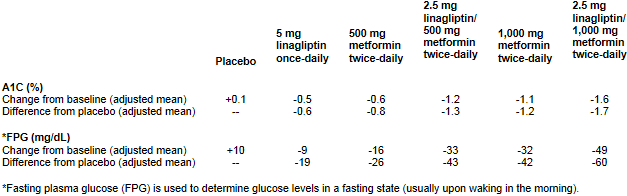
JENTADUETO was approved based on clinical trials that evaluated linagliptin and metformin as separate tablets. Bioequivalence of JENTADUETO was demonstrated with co-administered linagliptin and metformin tablets in healthy subjects.
Adverse reactions reported in greater than or equal to five percent of patients treated with JENTADUETO and more commonly than in patients treated with placebo included nasopharyngitis (the common cold) and diarrhea. Hypoglycemia was more commonly reported in patients treated with the combination of JENTADUETO and sulfonylurea compared with those treated with the combination of placebo, sulfonylurea and metformin (22.9 percent versus 14.8 percent, respectively). Pancreatitis was reported more often in patients randomized to linagliptin (1 per 538 person years versus zero in 433 person years for comparator).
“We are proud to bring this important, new, single-tablet treatment option, taken twice-daily, to the millions of people currently living with type 2 diabetes,” said David Pass, PharmD, vice president, cardiovascular and metabolic disorders marketing, Boehringer Ingelheim Pharmaceuticals, Inc. “We are hopeful that JENTADUETO, the newest member of the growing family of products from the Boehringer Ingelheim and Lilly diabetes alliance, may help people living with blood sugar levels that are not controlled.”
The FDA approval of JENTADUETO marks the second U.S. approval since the formation of the Boehringer Ingelheim and Lilly worldwide diabetes alliance in January 2011. The alliance leverages the collective scientific expertise and business capabilities of two leading research-driven pharmaceutical companies to address patient needs arising from the growing global diabetes epidemic.
What is JENTADUETO?
JENTADUETO is a prescription medicine that contains 2 diabetes medicines, linagliptin and metformin. JENTADUETO can be used along with diet and exercise to help control blood sugar in adults with type 2 diabetes when treatment with both linagliptin and metformin is appropriate.
JENTADUETO is not for people with type 1 diabetes or for people with diabetic ketoacidosis (increased ketones in the blood or urine).
It is not known if JENTADUETO is safe and effective when used with insulin.
References
- Centers for Disease Control and Prevention. National Diabetes Fact Sheet 2011. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed on: January 23, 2012.
- International Diabetes Federation. Diabetes Atlas Fact Sheet. 5th edn. Brussels: International Diabetes Federation, 2011.
- International Diabetes Federation. Diabetes Atlas. 3rd edn. Brussels: International Diabetes Federation, 2006.









