MERS vaccine shows promise in clinical trial, say researchers
Posted: 27 April 2020 | Victoria Rees (European Pharmaceutical Review) | No comments yet
A clinical trial to study a MERS vaccine has demonstrated success, say researchers, suggesting their technology could be repurposed to halt the spread of COVID-19.

A clinical trial to study a Middle East respiratory syndrome (MERS) vaccine has revealed positive results, say a team of scientists led by researchers at the German Centre for Infection research (DZIF). Currently, neither an effective vaccine against the MERS coronavirus nor a specific drug exist.
“The results of this vaccine trial are important and promising with regard to the development of a vaccine against SARS-CoV-2, the new coronavirus,” said Professor Marylyn Addo, Head of the Division of Infectious Diseases at the University Medical Center Hamburg-Eppendorf (UKE) and scientist at the DZIF. “The development of the MERS vaccine provides a basis upon which we at the DZIF can rapidly develop a vaccine against the new coronavirus.”
In 2014, the researchers began to develop a vaccine against the MERS coronavirus in preparation for large outbreaks of the virus. The vaccine is based on an attenuated virus (MVA: modified vaccinia virus Ankara), which had previously been used in a smallpox vaccination and has now been altered to contain protein components from the MERS coronavirus. This recombinant, vector-based vaccine is scientifically termed MVA-MERS-S. The MVA vector now serves as a basis for developing a vaccine against SARS-CoV-2, the new coronavirus.
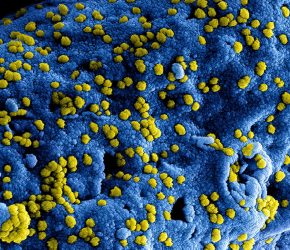

Digitally colorised scanning electron microscopic (SEM) image of MERS coronavirus particles [credit: NIAID].
In the current study, a total of 23 healthy trial volunteers were vaccinated twice with MVA-MERS-S, the experimental vaccine, with an interval of four weeks between the vaccinations.
“The tolerability and safety of the vaccine candidate as well as the resulting immune responses are very promising,” explained Dr Till Koch, one of the first authors of the trial. The vaccine was well tolerated. Local side effects occurred most frequently and presented in 69 percent of the trial subjects. No severe side effects occurred. “After the second injection of MVA-MERS-S, antibody formation and T cell responses occurred in 87 percent of the trial subjects,” summarised first co-author Dr Christine Dahlke.
Next, a Phase Ib trial, funded by the Coalition for Epidemic Preparedness Innovation (CEPI), will be conducted in which the vaccine will be tested in 160 trial subjects. At the DZIF, the results and tests from this trial will be used to start the development of a vaccine against the new coronavirus as rapidly as possible. The scientists will use the same viral vector (MVA) into which they will insert a SARS-CoV-2 Spike (S) protein to replace the MERS-CoV S protein.
The study was published in The Lancet.
Related topics
Clinical Development, Clinical Trials, Immunisation, Research & Development (R&D), Vaccine Technology, Vaccines, Viruses
Related organisations
German Centre for Infection research (DZIF), University Medical Center Hamburg-Eppendorf (UKE)
Related drugs
Related people
Related diseases & conditions
Coronavirus, Covid-19, Middle East Respiratory Syndrome (MERS)









