COVID-19 convalescent plasma therapy shown to be safe and effective
Posted: 3 June 2020 | Hannah Balfour (European Pharmaceutical Review) | No comments yet
Scientists reveal 76 percent of critically ill trial participants treated with convalescent plasma are recovering, with almost half now discharged from hospital.
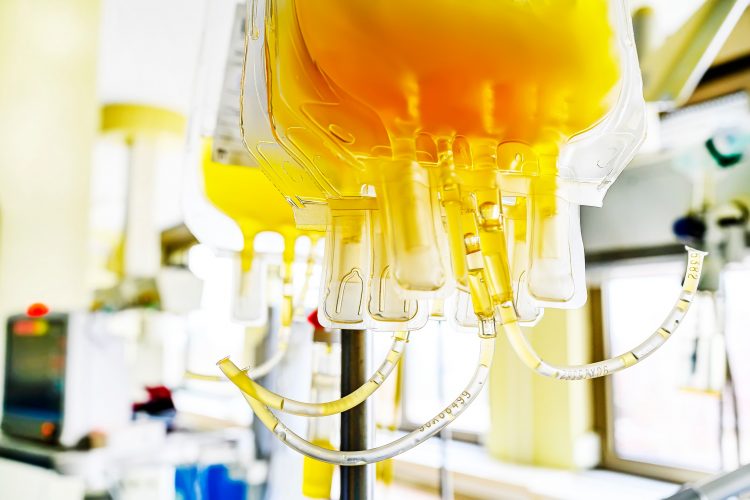

The largest COVID-19 convalescent plasma transfusion trial results have peer-reviewed and published, with 19 out of 25 (76 percent) of patients improving and 11 being discharged from hospital.
The Houston Methodist Hospital, US, first transfused plasma from recovered COVID-19 patients into two critically ill patients on 28 March, when it was available under US Food and Drug Administration (FDA) emergency use guidelines (Emergency Investigational New Drug [eIND]). However, after no adverse side effects were observed with the therapy, the FDA approved a trial of convalescent plasma transfusion as an investigational new drug (IND) seven days later.
“While physician scientists around the world scrambled to test new drugs and treatments against the COVID-19 virus, convalescent serum therapy emerged as potentially one of the most promising strategies,” said study corresponding author, Dr James Musser, chair of the Department of Pathology and Genomic Medicine at Houston Methodist. “With no proven treatments or cures for COVID-19 patients, now was the time in our history to move ahead rapidly.”
Convalescent plasma therapies have been used as early as 1918, when it was leveraged against the Spanish Flu, it has more recently been used during the 2003 Severe Acute Respiratory Syndrome (SARS) pandemic, the 2009 influenza H1N1 pandemic and the 2015 Ebola outbreak in Africa. After small studies indicated this therapy could benefit critically ill COVID-19 patients, an interdisciplinary team of Houston Methodist physician scientists and health care workers targeted COVID-19 with convalescent plasma therapy.
According to the team, the patient outcomes following plasma therapy were similar to those of patients treated with the antiviral remdesivir under the compassionate-use program. They concluded that any observed complications were consistent with COVID-19 disease progression and not caused by the transfusion of plasma.
The hospital is now considering conducting a randomised controlled trial to look at variables such as timing of the transfusion after the onset of symptoms, the number and volume of transfusions adjusted for patient biometrics, antibody levels in donor plasma and other parameters that could optimise the therapy and its use.
Since March a total of 74 critically ill COVID-19 patients have been infused with convalescent plasma, not all were part of this trial. Of those treated, 50 have been discharged from hospital.
The study authors also revealed more than 150 individuals who had recovered from COVID-19 infections donated their plasma.
The peer-reviewed findings were published in The American Journal of Pathology.
Related topics
Clinical Trials, Drug Safety, Regulation & Legislation, Research & Development (R&D), Therapeutics, Viruses
Related organisations
Houston Methodist Hospital, US Food and Drug Administration (FDA)
Related drugs
Related people
Related diseases & conditions
Coronavirus, Covid-19, Ebola virus, Influenza, Severe Acute Respiratory Syndrome (SARS), Spanish influenza