Thermo Fisher Scientific handheld instruments selected by USP for spectral library development project
Posted: 14 January 2013 | Thermo Fisher Scientific | 1 comment
Thermo Fisher Scientific Inc.,the world leader in serving science, announced that the United States Pharmacopeial Convention (USP) has selected its Thermo Scientific TruScan RM and microPHAZIR RX instruments to support its recent initiative to provide USP-approved libraries to pharmaceutical manufacturers.


Thermo Scientific TruScan RM
This spectral library project is one of the many USP initiatives designed to set standards that help ensure the quality, safety and benefit of medicines and foods. With an assembled consortium of world class vendors, this project aims to provide manufacturers with a USP-approved library of methods for use across the globe for material identification. This provides a harmonized approach to ensuring the quality of pharmaceutical ingredients before entering the supply chain.
“With increases in supply chain variability, the development and deployment of public standards is critical to ensure the safety and efficacy of medicines,” said Edward Zhao, vice president, business development of USP. “USP relies on advanced analytical capabilities and modern equipment, such as the TruScan RM and microPHAZIR RX, to provide the highest quality of standards for use by global pharmaceutical manufacturers.”
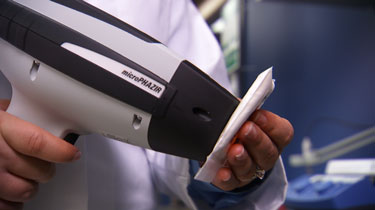

Thermo Scientific microPHAZIR RX
TruScan RM, a Raman-based spectrometer, and microPHAIR RX, based on near-infrared (NIR), will be used to develop all methods included in the USP spectral library. Both instruments are easy to use and enable on-the-spot, actionable pass-fail results within seconds.
“We believe it’s important to provide tools to our customers that support their mandates for enhanced product quality and a more secure supply chain,” says Maggie Pax, senior director, Pharma-Chem, Thermo Fisher portable analytical instruments. “Our TruScan RM and microPHAZIR RX instruments are perfect complements to the library development project, providing USP with a rapid, easy to use way to develop methods that can be quickly deployed to manufacturers around the world.”
About TruScan RM and microPHAZIR RX
Developed with in-depth pharmaceutical industry experience, Thermo Scientific handheld Raman and NIR analyzers provide manufacturers with a portable solution to achieve quality initiatives throughout the manufacturing process. Easily operated by non-expert users, the analyzers perform non-destructive analysis and can operate through plastic or glass containers to enable immediate release of raw materials into production. With greater than 600 GMP installations worldwide, both analyzers are designed to enable customers to achieve compliance with 21 CFR Part 11 and meet the requirements of global pharmacopeias.
For more detailed information about Thermo Scientific handheld analytical instruments, visit http://www.thermoscientific.com/quality.





About the spectral libraries for the hand held raman spectrometers, are they specific for APIs or can they be used for finished product as well; and if so how reliable can the results be?