First-of-a-kind psoriasis study outcomes revealed
Posted: 24 January 2024 | Catherine Eckford (European Pharmaceutical Review) | No comments yet
Phase III data for Janssen’s innovative IL-23 inhibitor has revealed that the biologic is effective for adults with moderate to severe plaque psoriasis (PsO) across all skin tones.
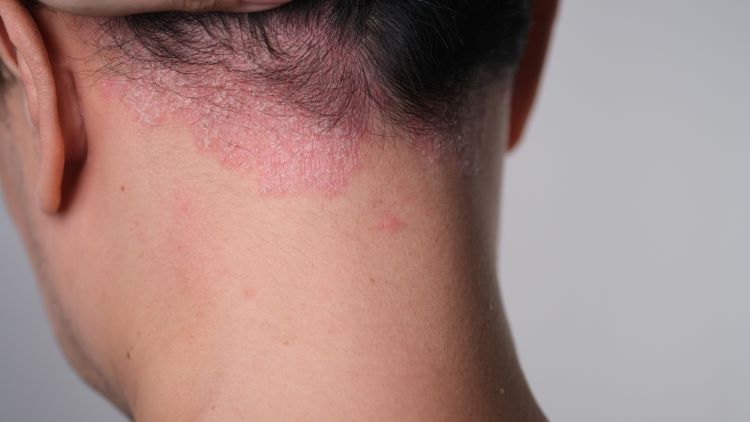

Topline data from a novel Phase III study show that the biologic TREMFYA ® (guselkumab) facilitated rapid and significant clearance in moderate to severe scalp psoriasis (PsO) and significant improvement in scalp itch at 16 weeks, according to Johnson & Johnson.
It is the first prospective, large-scale, randomised-controlled clinical trial evaluating people of colour across all skin tones with moderate to severe plaque PsO and scalp PsO to objectively measure clearance and other treatment outcomes with Tremfya.
Johnson & Johnson emphasised that Tremfya, developed by Janssen, is the first IL-23 inhibitor authorised in the US to treat adults with moderate to severe plaque PsO who are candidates for systemic therapy or phototherapy as well as adults with active psoriatic arthritis (PsA).
[In Cohort B of the VISIBLE trial] a nearly 90 percent improvement (87.6 percent compared to 37.8 percent placebo) was observed from baseline in Psoriasis Scalp Severity Index (PSSI) and in Scalp Surface Area (SSA) (86.6 percent versus 33.4 percent for placebo)”
What the Phase IIIb study of Tremfya found
The topline data from Cohort B of the Phase IIIb VISIBLE study presented at the Maui Derm Hawaii 2024 conference, showed that at Week 16, after three doses of Tremfya, patients achieved significantly greater improvements versus placebo.
A nearly 90 percent improvement (87.6 percent compared to 37.8 percent placebo) was observed from baseline in Psoriasis Scalp Severity Index (PSSI) and in Scalp Surface Area (SSA) (86.6 percent versus 33.4 percent for placebo).
Importance of the data
“Scalp psoriasis is highly prevalent and can present unique challenges across diverse populations. In patients of colour, cultural factors related to hair care practices and post-inflammatory pigment changes contribute to the burden of scalp psoriasis,” shared Dr Andrew Alexis, MPH, Professor of Clinical Dermatology and Vice Chair for Diversity and Inclusion at Weill Cornell Medicine in New York and Lead Study Investigator.
The combined data from Cohort A and Cohort B from the VISIBLE study assessing Tremfya is a key part in helping this patient population, because “people of colour continue to experience undertreatment, with many participants not receiving a biologic option prior to this trial enrolment,” commented Jennifer Davidson, DO, Vice President of Medical Affairs, Immunology at Johnson & Johnson Innovative Medicine.
“These findings from VISIBLE are key to broadening our understanding of scalp psoriasis, and the role Tremfya can have in treating all patients with this disease,” Dr Alexis added.
Johnson & Johnson confirmed that study will evaluate treatment of Tremfya in approximately 211 participants from the US and Canada, who will be followed for approximately two years.
Related topics
Big Pharma, Biologics, Biopharmaceuticals, Clinical Development, Clinical Trials, Drug Development, Drug Safety, Industry Insight, Research & Development (R&D), Therapeutics









