Bacteria therapy shows promise for eczema
Posted: 7 May 2018 | Dr Zara Kassam (European Pharmaceutical Review) | No comments yet
Topical treatment with live Roseomonas mucosa was found to be safe for adults and children with atopic dermatitis…
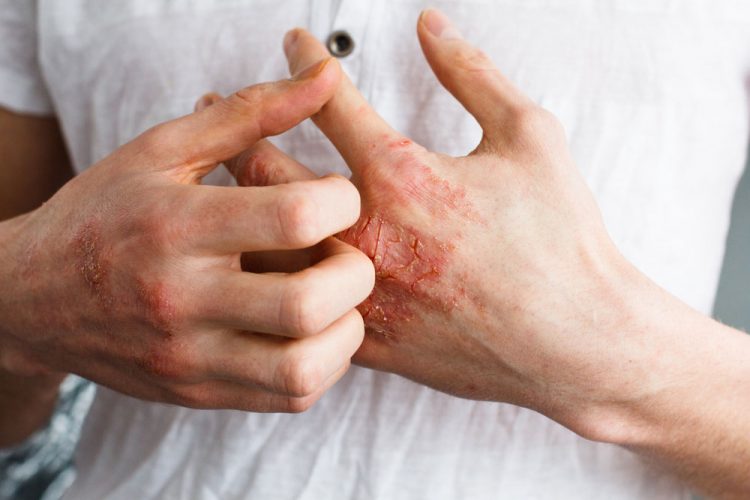

Topical treatment with live Roseomonas mucosa — a bacterium naturally present on the skin — was safe for adults and children with atopic dermatitis (eczema) and was associated with reduced disease severity, according to initial findings from an ongoing early-phase clinical trial at the National Institutes of Health. Preclinical work in a mouse model of atopic dermatitis had suggested that R. mucosa strains collected from healthy skin can relieve disease symptoms.
Atopic dermatitis is an inflammatory skin disease that can make skin dry and itchy, cause rashes and lead to skin infections. The disease is linked to an increased risk of developing asthma, hay fever and food allergy. Atopic dermatitis is common in children and sometimes resolves on its own, but it also can persist into or develop during adulthood.
“Living with atopic dermatitis can be physically and emotionally challenging. While treatment can help manage the symptoms, currently available therapies can be time-consuming — requiring multiple daily applications — and costly,” said Dr Anthony S. Fauci, director of NIH’s National Institute of Allergy and Infectious Diseases (NIAID). “New, inexpensive therapies that require less frequent application are needed to expand the options available for atopic dermatitis treatment.”
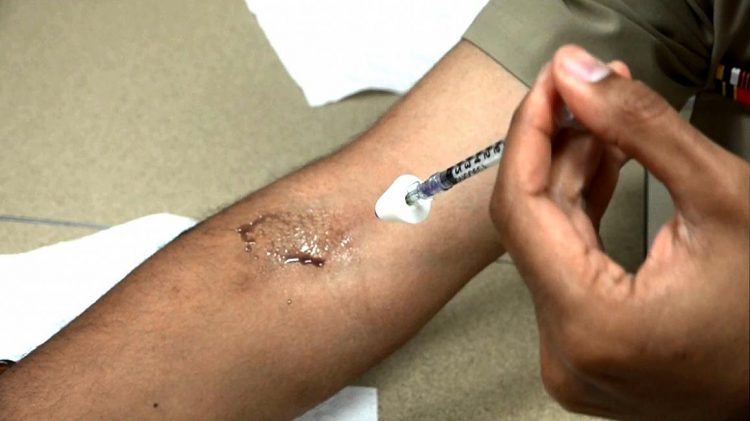

A scientist demonstrates application of the experimental therapy to the inner elbow. For demonstration purposes, the bacteria solution has been replaced with purple dye. (NIAID)
The cause of atopic dermatitis is unknown, but studies suggest that the skin microbiome—the community of bacteria and other microbes living on the skin—plays a key role. For years, scientists have known that people with atopic dermatitis tend to have large populations of Staphylococcus aureus bacteria on their skin. These bacteria can cause skin infections and trigger immune responses that increase inflammation and worsen symptoms.
Recent work by NIAID researchers using a mouse and cell culture models of atopic dermatitis revealed that treatment with isolates of R. mucosa collected from the skin of healthy people improved disease outcomes in the models. In contrast, R. mucosa isolates from people with atopic dermatitis either had no impact or worsened outcomes in the models.
Based on these preclinical findings, the investigators designed an early stage clinical trial to test the safety and potential benefit of a treatment containing live R. mucosa in people with atopic dermatitis. The Phase 1/2 study is now being conducted at the NIH Clinical Center in Bethesda, Maryland.
“By applying bacteria from a healthy source to the skin of people with atopic dermatitis, we aim to alter the skin microbiome in a way that will relieve symptoms and free people from the burden of constant treatment,” said NIAID’s Dr Ian Myles, the principal investigator of the trial. “If future clinical studies demonstrate that this strategy is effective, we hope our work will lead to the development of new, low-cost atopic dermatitis therapies that do not require daily application.”
The researchers first tested the experimental treatment in 10 adult volunteers with atopic dermatitis. Twice a week for six weeks, the volunteers sprayed a solution of sugar water containing increasing doses of live R. mucosa onto their inner elbows and one additional skin area of their choice. The R. mucosa strains included in the treatment were originally isolated from the skin of healthy individuals and grown under carefully controlled laboratory conditions. Participants were instructed to continue their normal eczema treatments, including topical steroids and other medications.
Participants did not report any adverse reactions or complications. Most participants experienced improvements in their atopic dermatitis, and four weeks after stopping the bacteria therapy, some reported needing fewer topical steroids.
The investigators next enrolled five volunteers aged 9 to 14 years with atopic dermatitis. Treatments were applied to all affected skin areas twice weekly for 12 weeks and every other day for an additional four weeks. Consistent with the findings in adults, there were no complications or adverse effects, and most participants experienced improvements in their eczema, including a reduced need for topical steroids. The researchers also found that treatment was associated with decreases in the S. aureus population on the children’s skin.
Although larger studies comparing the bacteria therapy with a placebo will be required to assess the effectiveness of this potential treatment, the investigators observed a greater than 50 percent improvement in atopic dermatitis severity in four of the five children and six of the 10 adults. The researchers are continuing to monitor the five children who received treatment and are enrolling additional children into the study.
To better understand factors that may contribute to imbalances in the bacteria on the skin, the scientists also investigated whether chemicals produced by R. mucosa or present in certain skin products may be associated with atopic dermatitis. They found that strains of R. mucosa from people with atopic dermatitis produced skin irritants, while strains isolated from healthy skin produced chemicals that may enhance the skin’s barrier and help regulate the immune system. In addition, some forms of parabens—a common preservative in skin products—and some topical emollients (moisturizers) blocked the growth of R. mucosa from healthy skin and did not have as strong an effect on growth of S. aureus or eczema-associated R. mucosa. These findings suggest that certain products may worsen atopic dermatitis and/or affect the effectiveness of microbiome-based therapies.
Final results from the ongoing study at NIH will provide the foundation for larger trials to evaluate the efficacy of this novel investigational therapy, as well as to better understand the role of R. mucosa in atopic dermatitis.
The findings have been published in JCI Insight.
Related topics
Drug Delivery Systems, Drug Development, Drug Targets, Microbiology, Microbiomes, Research & Development (R&D)







![Roche logo sign lit up [Credit: testing/Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Roche-3-400x187.jpg)

