AbbVie JAK inhibitor shows potential in novel atopic dermatitis head-to-head trial
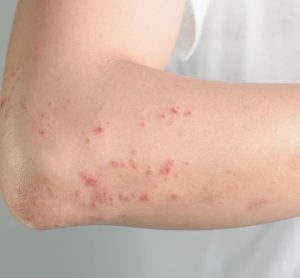
Patients with moderate-to-severe atopic dermatitis can strive for both little to no itch and clearer skin with AbbVie’s upadacitinib, topline Phase IIIb/IV study results suggest.