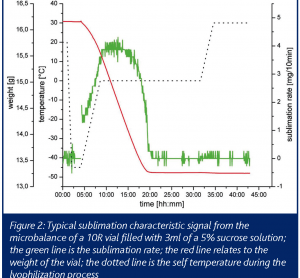
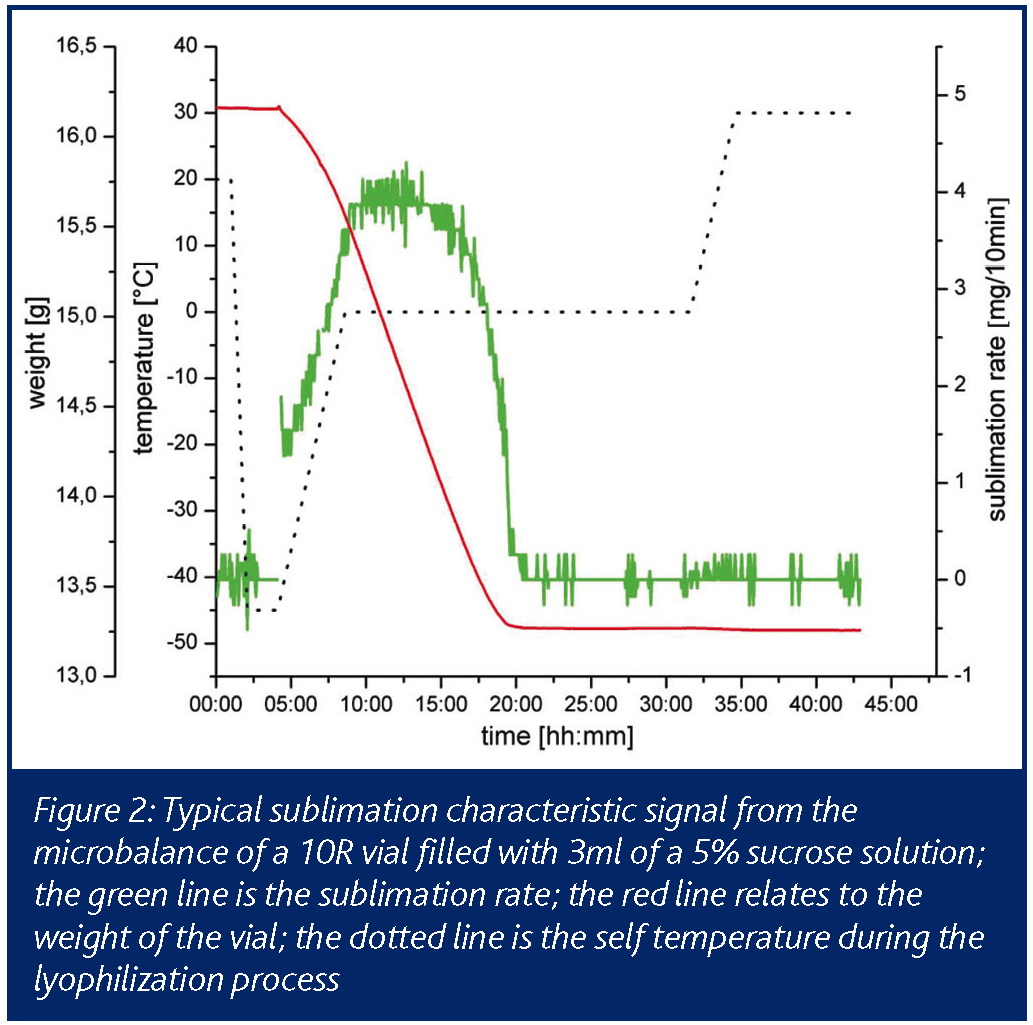
PAT for freeze drying: cycle optimisation in the laboratory
25 January 2007 | By Dr. Henning Gieseler, PhD., Department of Pharmaceutics, University of Erlangen-Nuremberg
Freeze drying is generally known to be a time consuming and therefore expensive process. In order to lower costs during manufacturing, the effective cycle time must be reduced. This goal can be achieved by optimising a freeze drying cycle in the laboratory – in particular the primary drying phase. Applying…