International standards: a tool for compliance
Posted: 22 August 2005 | | 1 comment
Regulatory guidance documents, such as Annex 1 to the GMP guideline of the European Union1 and FDA’s comparable Guidance for Industry2 establish the objectives to be met by pharmaceutical contamination control systems – especially those for the production of sterile medicinal drugs.
Regulatory guidance documents, such as Annex 1 to the GMP guideline of the European Union1 and FDA's comparable Guidance for Industry2 establish the objectives to be met by pharmaceutical contamination control systems – especially those for the production of sterile medicinal drugs.
Regulatory guidance documents, such as Annex 1 to the GMP guideline of the European Union1 and FDA’s comparable Guidance for Industry2 establish the objectives to be met by pharmaceutical contamination control systems – especially those for the production of sterile medicinal drugs.
For the details to be observed for ensuring performance and quality during design, construction, start-up, qualification and operation of pharmaceutical cleanroom systems, these guidance documents rely upon – and make explicit reference to – technical standards. Here, the international standards developed under the auspices of ISO – the International Organization for Standardization – merit particular consideration.
EN ISO families of standards
Supported by CEN – the European Committee for Standardization – ISO has embarked, in 1993, upon the development of two families of contamination control standards:
- the EN ISO 14644 series on cleanroom technology in general3
- the EN ISO 14698 family on biocontamination control4
EN ISO standards are developed in a sequence of steps. Point of departure is a New Work Item Proposal NWIP, which requires active support from a specified number of nations committing themselves to participate in the elaboration of the forthcoming standard. The approval procedure is subdivided into three stages in sequence:
- an informal circulation as Committee Draft CD, with the objective of inviting technical comments from the nations actively involved in the development of the standard
- a first formal circulation as Draft International Standard DIS for the parallel ISO and CEN enquiries, inviting technical and editorial comments and requesting a generic statement on the merits of the draft by means of a preliminary vote
- and finally the second formal circulation as Final Draft International Standard FDIS, for the parallel ISO and CEN voting leading – if successful – to approval and subsequent publication in the ISO and CEN collections of standards
Thereafter, all CEN nations are required to incorporate them – within six months – into their national collections of standards. Thus, publication in 28 national standards collections is guaranteed right from the beginning – strong motivation indeed for worldwide adoption and acceptance. In parallel, CEN members are required to withdraw any national standards on the same subject matter.
Five years after publication in the ISO and CEN collection of standards, each standard is automatically submitted to Systematic Review and subsequently corrected or amended if this is deemed necessary.
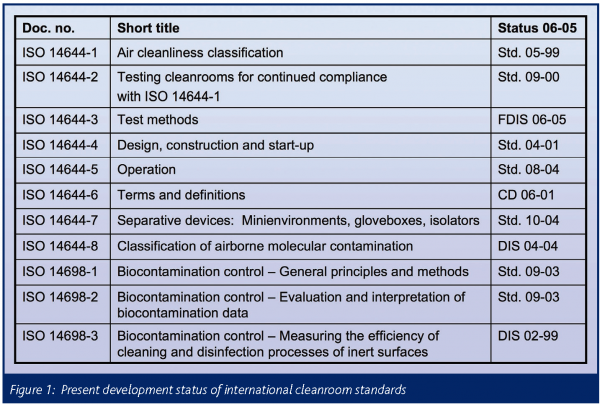
A compilation of the standards already issued and under development is presented in Figure 1; the column status 06-05 identifies their actual state of development. In addition to these work items, the elaboration of an international standard devoted to the important issue of surface cleanliness has recently been initiated. First circulation as Committee Draft is imminent.
Impact upon industry
From DIS status onwards, ISO standards:
- can be purchased through the national standardisation bodies and their sales outlets
- can be used as reference documents and as base documents for projects and customer/supplier agreements
- are considered to represent the state-of-the-art in any legal dispute
As soon as they have reached DIS status, international standards should be given priority over national standards and guidelines and recommended practices elaborated by professional societies. With ten international cleanroom standards now in the DIS stage and beyond, ample guidance is now available for most contamination control topics of interest to the pharmaceutical industry.
Air cleanliness classification
For more than 30 years, U.S. Federal Standard 2095 had been the leading standard worldwide for classifying cleanrooms. The latest edition, 209E, published in 1992, was officially withdrawn in 2001 and replaced by the corresponding ISO standard.
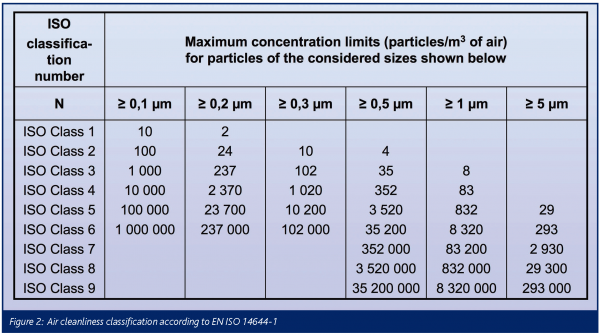
The air cleanliness classification scheme according to EN ISO 14644-1 is based upon a formula and shown in Figure 2 in tabular form. The range of particle diameters for air cleanliness classification extends from 0.1 to 5μm. Thus, it is focussed upon the detection capabilities of the discrete-particle counter, the reference test method for demonstrating compliance with EN ISO 14644-1. Tests shall be conducted with calibrated instruments and class limits must be met with the rigours of the 95 per cent upper confidence limit.
The remaining parts of the series
The following information focuses upon the standards of direct relevance for the pharmaceutical industry (see lit. 6 for more details).
EN ISO 14644-2 establishes the minimum requirements for reclassification and re-qualification work, as well as the minimum time intervals between subsequent reclassifications. During periodical requalification, tests should comprise at least:
- confirmation of the air cleanliness class
- pressure differentials between rooms
- verification of the air flow rate (with turbulent airflow) or the air velocity (with unidirectional airflow)
In the case of pharmaceutical production facilities, the optional leak test of the installed terminal HEPA filters protecting the grade A areas is also strongly recommended. Extraordinary requalification should be performed after significant changes to the installation and after major maintenance operations (e.g. replacement of the terminal HEPA filters).
EN ISO 14644-3 – presently at the FDIS stage – provides guidance for tests and measurements of the physical parameters relevant in the cleanroom context. A total of 14 measuring tasks are addressed. In addition, the minimum performance requirements for the test instrumentation are specified in detail.
ISO 14644-4 offers comprehensive and systematic guidance for design, construction and start-up of cleanrooms; it may well be perceived as the basic compendium of cleanroom design and realisation. One of the recommendations to be highlighted is the shell concept as a zoning principle for clean facilities (Figure 3). It illustrates transparently how contamination transport towards the critical areas can be dominated effectively by gradually increasing the contamination control requirements from zone to zone. Limiting the core area with the highest air cleanliness requirements to the absolute minimum also addresses the economic issue of cost minimisation in investment and operation. ISO 14644-4 provides for the first time concise guidance for the qualification checks and tests to be performed during the subsequent realisation stages of a clean facility. Worth mentioning, moreover, is a systematic compilation of all the determinations having to be agreed between purchaser/user and designer/supplier during project development. It is condensed into a comprehensive checklist covering 12 subject areas with a grand total of 151 items! If project design is cross-checked with its help, it is almost impossible to have overlooked something of importance.
EN ISO 14644-5 addresses the operational aspects of cleanrooms with a focus on the principal hazards: cleanroom clothing, personnel, stationary equipment, materials as well as portable and mobile equipment and cleanroom cleaning.
EN ISO 14644-7 establishes requirements for separative enclosures, i.e. clean air hoods, glove boxes, isolators and mini-environments. Guidance is limited to issues not covered in EN ISO 14644-3 and 14644-4, for example isolator-specific measurement tasks such as integrity testing of containments and glove-sleeve systems.
Biocontamination control standards reviewed
EN ISO 14698-1 is devoted to the general principles and the measurement of biocontamination. It provides guidance on the measurement of airborne biocontamination and on microbiological sampling of surfaces, textiles and liquids. It also covers the validation of air sampling and laundering processes, as well as the subject of personnel training.
EN ISO 14698-2 on evaluation and interpretation of biocontamination data distinguishes between the requirements for the initial monitoring phase, when procedures are set up, tested, evaluated and validated and those for handling biocontamination data resulting from routine process monitoring.
Surface cleanliness
Surface cleanliness is the only pharmaceutically important subject area not yet addressed in the EN ISO 14644 series. For this topic, however, an excellent alternative compendium can be recommended: the guideline VDI 2083 Part 47 elaborated by VDI (Verein Deutscher Ingenieure = Association of German Engineers). Published bilingually (left columns German, right columns English), this compendium covers its topic most comprehensively, specifically taking pharmaceutical aspects into consideration. It is one of the basic documents instrumental for development of the forthcoming EN ISO 14644-9 standard on surface cleanliness.
Air cleanliness in GMP compendia
Both FDA and the European Community establish room grades for aseptic processing (FDA2) or for the production of sterile medicinal products in general (the European approach1). How well do these pharmaceutical room grades correlate with the air cleanliness classes as defined in EN ISO 14644-1?
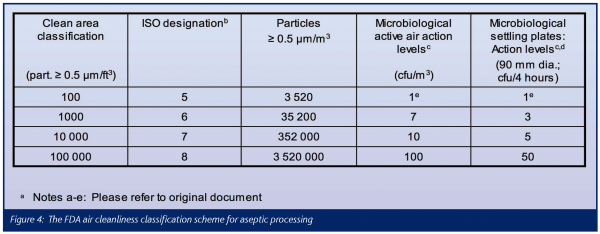
The FDA air cleanliness classification scheme for aseptic processing is reproduced in Figure 4. Particle concentration limits are established for particles > 0.5μm and above only and they are strictly based upon – and referenced to – ISO 14644-1. The denomination of the air cleanliness classes, however, follows the traditional American practice, with the cu.ft. as volume unit (example: Class 100 = 100 particles > 0.5 μm/cu.ft. at class limit = 3 520 particles > 0.5 μm/m3 at class limit = ISO Class 5). The room air cleanliness requirements based upon this scheme apply in the occupancy state operational.
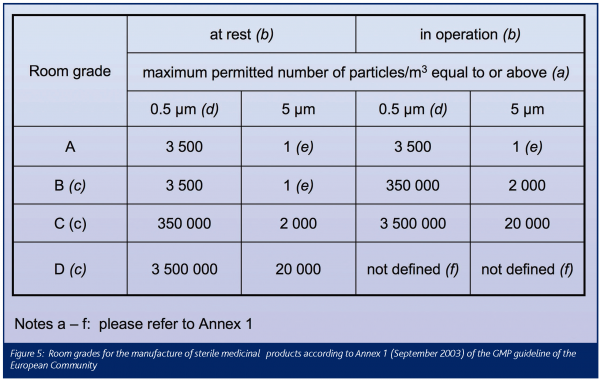
Annex 1 to the European GMP guideline establishes particle concentration limits for two occupancy states: at rest and operational (Figure 5). For particles 0.5mm and above, the class limits comply with EN ISO 14644-1. This is, however, not at all true for particles 5mm and above! For room grades C and D, the differences in the limits are reasonably minor. For room grade A and B in the at rest occupancy state, however, the differences are substantial. Corresponding with ISO Class 3 (sic!), they are extremely strict: two classes stricter than those applying for particles 0.5mm where ‘only’ ISO class 5 is to be met. European industry is extremely unhappy about these 5mm determinations and most disappointed that their regulators continue to ignore the ISO air cleanliness determinations8-11.
International harmonisation – wishful thinking?
Comparing the attitudes behind the new regulatory documents for sterile manufacturing, different philosophies seem to be followed on both shores of the Atlantic. The openness to dialogue with industry so apparent in recent FDA policy and in the new-generation FDA guidance documents is not yet reflected by the European regulators. Even worse: the European and American GMP requirements for aseptic processing and the manufacture of sterile medicinal products seem to drift more and more apart. If both authorities have to be satisfied – and this is nowadays the normal situation – extra effort and, as a consequence, extra cost is incurred.
The argument is frequently heard that both GMP guidelines are 99 per cent harmonised. However, in the remaining 1 per cent of discrepancies there are most relevant ones to be found, such as the European determinations for particles > 5μm!
The international cleanroom standards have prepared the path: within a reasonably short period in time, generally accepted standard families have been elaborated. Moreover, international co-operation has set quality benchmarks: ISO 14644-1 on air cleanliness classification, for example, is generally perceived as a step forward compared to its predecessors, especially U.S. Federal Standard 209E.
Therefore, there is no reason for international harmonisation of the world’s GMP compendia to remain wishful thinking. An international GMP guideline is a realistic goal – a goal worth striving for!






References
- Ad Hoc GMP Inspections Services Group: EC Guide to Good Manufacturing Practice – Revision to Annex 1: Manufacture of sterile medicinal products. European Commission, Brussels, 30 May 2003.
- Guidance for industry: Sterile drug products produced by aseptic processing – current Good Manufacturing Practice. U.S. Department of Health and Human Services, Food and Drug Administration (September 2004).
- EN ISO 14644. Cleanrooms and associated controlled environments. International Organization for Standardization ISO, Geneva and European Committee for Standardization CEN, Brussels; see Figure 1 for details.
- EN ISO 14698. Cleanrooms and associated controlled environments – Biocontamination control. International Organization for Standardization ISO, Geneva and European Committee for Standardization CEN, Brussels; see Figure 1 for details.
- U.S. Federal Standard 209E: Airborne particulate cleanliness classes in clean rooms and clean zones. Washington DC/USA (11 September 1992, withdrawn 29 November 2001).
- Schicht H.H.: The ISO contamination control standards – a tool for implementing regulatory requirements. Eur. J. Pharm. & Parenteral Sci. 8 (2): 37-42 (2003).
- VDI 2083 Part 4: Cleanroom technology – Surface cleanliness. Beuth-Verlag, Berlin (February 1996).
- Farquharson G.: EU GMP Annex 1 – ISO 14644-1 – FDA aseptic guidance: Is this a good basis for an international sterile products GMP? Eur. J. Pharm. & Parenteral Sci. 9 (4): 133-138 (2004).
- Fairchild St.: May 2003 revision of the Annex 1 to the EU GMP guide. GMP Review 2 (3): 5-7 (2003).
- Schicht H.H.: The revised Annex 1 to the EC GMP guideline. GMP Review 2 (3): 7-11 (2003).
- Schicht H.H.: Die neuen europäischen und amerikanischen Regularien für die Sterilherstellung – eine kritische Stellungnahme (The new regulatory guidance in Europe and the USA for sterile manufacturing – a critical assessment). Swiss Pharma 26 (7-8): 9-21 (2004).









[…] Incorporate ISO standards and look for FSMA compliance methods toolbox. Don’t wait for the FDA to put these rules and make them into regulations before you start complying with them. Also, you can work with strict rules that govern medicinal compounds with the perception that CBD derived from hemp applications are in the medical industry. FSMA indicates where the plant is making that product grow, whose farm, growing conditions and the quality of the final product. […]