Tc-99m radiopharmaceuticals and in-house chromatographic methods
Posted: 20 November 2019 | Anastasia Petropoulou (University Hospital Bristol’s NHS Foundation Trust) | No comments yet
Technetium-99m (99mTc) is a synthetic radioisotope that is used worldwide to produce radiopharmaceuticals that are mainly used for diagnostic purposes in nuclear medicines. The purity of the final radiopharmaceutical (99mTc-R) can be confirmed by applying chromatographic methods. This method is based on different rates of movement of substances in a sample solution through a porous medium under the influence of a reagent (either a solvent or gas). Anastasia Petropoulou explores some chromatographic techniques used to identify impurities in radiopharmaceuticals based on 99mTc.

Radiopharmaceuticals and their use
The term radiopharmaceutical as it is accepted today covers over a hundred simple and complex radioactive chemicals used in in vivo diagnostic/therapeutic applications and for in vitro clinical diagnostic tests. They are defined as radionuclidic/labelled compounds intended for administration for therapeutic or diagnostic purposes.1 The European Pharmacopoeia mandates that all radioactive pharmaceuticals must be sterile, apyrogenic, free from extraneous particles and of a suitable pH. In addition, radioactive injections must be of the correct radiochemical and radionuclidic purity and have the correct radioactivity present at the stated time of injection. An unacceptable radiochemical purity (RCP) can lead to the radioactivity localising in an unintended target, which may result in organ damage.2 For this reason, the administrative departments of the health ministry responsible for pharmaceutical substances insist that radiopharmaceutical products should be subject to control and must satisfy the purity criteria laid down by the pharmacopoeia commissions aiming to protect public health.1
Purity criteria for radiopharmaceutical products
The purity criteria of radiopharmaceuticals will depend on the chemical processing undertaken during preparation of the radioactive product and on the pharmaceutical form in which the product is supplied. As an example: the purity of a sodium iodide (I131) solution without carrier, which contains only sodium carbonate and sodium iodide in very small quantities, will have greater purity than the gelatine capsule containing sodium iodide since it will contain impurities due to the gelatine colouring agent, antiseptic and reducing agents, which are added to preserve the dry state of the iodine in iodide form.2 Radiopharmaceuticals should pass the radionuclidic and radiochemical purity test before being administered to patients.
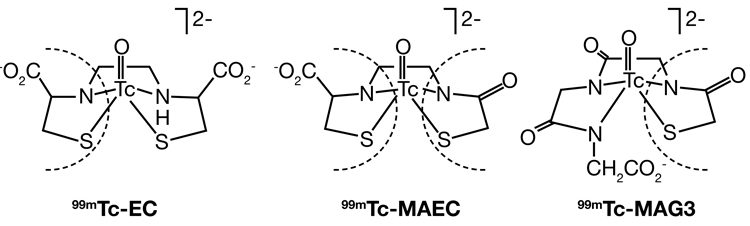

Figure 1: 99mTc-MAEC 99mTc-MAG3 and 99mTc-EC radiopharmaceuticals11
Radionuclidic purity is the amount of radioactivity, as a percentage, due to the radionuclide concerned compared to the measured activity of the radiopharmaceutical preparation. Radionuclide impurities can occur as a result of the manufacturing process. As an example: for nuclides produced by cyclotron there can be contaminants resulting from impurities in the target or by the energy of the reaction. Impurities can also arise due to the presence of the parent nuclide and this applies when the stated nuclide is obtained by a separation technique such as a generator elution; for example, the presence of Molybdenum (99Mo) in a 99mTc radiopharmaceutical. 99Mo in the labelled pharmaceutical compound can be detrimental to the patient due to the beta emission and its long half-life that gives an increased radiation dose.4
Radiopharmaceutical products should be subject to control and must satisfy the purity criteria laid down by the pharmacopoeia commissions aiming to protect public health”
RCP has been designed to ensure that the target radioisotope is attached to the ligand and is not free or attached to another chemical entity, as in this form it may have a different biodistribution in the body after injection of the radiopharmaceutical.4
Technetium-99m-labelled radiopharmaceuticals
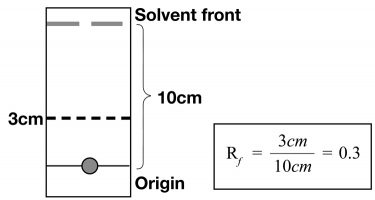

Figure 2: A complete chromatographic strip. The radiochemical component has migrated to 3cm above the origin when the solvent front has reached to 10cm on the strip. The Rf value is 0.3.2
Tc-99m is obtained daily by elution of the 99Mo‑99mTc generator with saline. In the elution process, TcO4– [Tc (VII) and possibly reduced forms such as Tc (VI), Tc(V) and Tc (IV)] are obtained.5 The reduced forms rarely cause contamination on the TcO4– radioisotope but when they are present will cause problems with imaging and in the preparation process of 99mTc-R.5 For most radiopharmaceuticals, the lower limit of RCP is 95 percent; that is, at least 95 percent of the radioactive isotope must be attached to the ligand. RCP determination can be carried out by a variety of chromatographic methods.2 Technetium-99m-labelled radiopharmaceuticals prepared from nonradioactive kits and TcO4– generally depend on the efficiency of the reducing agent in the 99mTc-R to reduce Tc (VII) to Tc (III), Tc (IV) or Tc (V). The reduced form of Tc-99m is labelled to a chelating agent or other organ-specific chemical.
When the reduction is incomplete, TcO4– [Tc (VII)] may be a radiochemical impurity in the Tc-99m-labelled/ final radiopharmaceutical. Unreduced TcO4– will not tag. The most common reducing agent is stannous ion.3
Radiochemical impurities in stannous-reduced Tc-99m radiopharmaceuticals are categorised as follows:
- Free Tc-99m, TcO4-, Tc (VII): stannous ions in the kit can become oxidised to stannic ion, Sn (IV) when air enters the vial. In this case, the radionuclide is no longer capable of reducing Tc (VII) to a lower oxidation state, which causes the non-reduced free technetium to become a chemical impurity, as it cannot tag to the chelate.2
- Technetium-99m-Tin colloid: stannous ions can become hydrolysed to stannous hydroxide owing to the presence of water; this causes the stannous hydroxide chemical impurity to bind to the reduced Tc-99m and forms a Tc-99m stannous hydroxide complex compound. The risk is the colloidal impurity in the kit to localise in the patient’s reticuloendothelial system.2
- Technetium-99m dioxide: Tc-99m reduced by the stannous ion in the kit can become hydrolysed to technetium dioxide before tagging. This water insoluble complex remains as a radiochemical impurity in the 99mTc-R radiopharmaceutical.2
- Re-oxidised Tc-99m: reduced Tc-99m that has already been tagged to a chelate can re-oxidise and revert to TcO4– and this rate of re-oxidation accelerates with an increase in the air content within the vial. The rate of re-oxidation can affect the expiration time of the Tc-99m labelled radiopharmaceutical. Re-oxidation can be minimised by avoiding the introduction of air into the vial and by packing the non-radioactive kits in a nitrogen atmosphere.6
- Bound Tc: the desired radiochemical species.
Technetium-99m-based radiopharmaceuticals and chromatography
Paper and Instant Thin-Layer Chromatography (ITLC) are the analytical methods mostly used to determine radiochemical purity in Tc-99m kits. Cellulose filter paper of chromatographic grade, ie, Whatman chromatography papers and glass microfibre impregnated with silica gel or silic acid5 are the supports for paper and ITLC, respectively.
Microlitre amounts of the radiopharmaceutical are applied to the solid subject, ie, a paper chromatographic strip or ITLC strip at a point near its bottom edge, called the origin.2 Chromatography is accomplished by placing the strip containing the origin into a suitable solvent in a chromatography chamber such that the spot on the origin is not immersed in the solvent/ mobile phase and the chamber is covered with a lid to maintain a solvent-saturated atmosphere. The solvent moves along the strip by adsorption and capillary action separating the components of the radiopharmaceutical. The electrostatic forces (adsorption) of the stationary phase retard the various radiochemical species while the mobile phase moves them along. This effect and the fact that the radiochemical species have different solubility in the mobile phase cause the species to move at different speeds.2,5
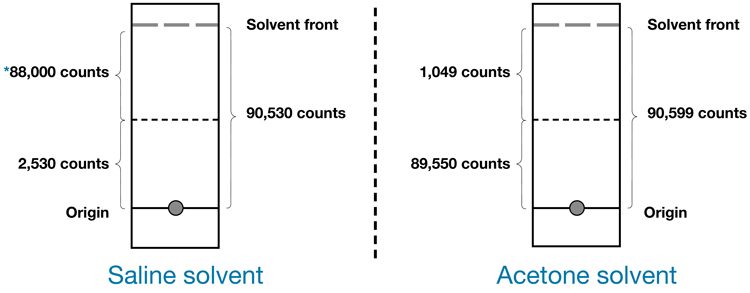

Figure 3: This illustrates a 99m-Tc-labelled compound soluble in saline and highlights the differences in counts
between two solvents used in the chromatographic process. The percentage of hydrolysed-reduced Tc-99m in the kit was determined by calculating the counts of each strip developed in a suitable mobile phase: percent hydrolysed Tc-99m = 2530/90,530 x 100 = 2.8 percent, 99mTcO4– = 1,049/90,530 x 100 = 1.2 percent.2,3
The most common chromatographic technique used includes paper and ITLC chromatography because these techniques provide the most accurate and fastest chromatographic results”
After the solvent front (Sf) moves the desired distance the strip is removed from the chamber and left to dry. The strip is then divided horizontally into equal segments and the radioactivity of each equal segment is measured with an appropriate counter; ie, well scintillation counter, etc. The percentage of radiochemical impurities is calculated from the ratio of the radioactivity (counts per second [cps]) associated with the impurity compared with the radioactivity of the total strip. Figure 3 represents the solvent front of Tc-99m-MAG3. The Sf on this example signifies the hydrophilic impurities, primarily free pertechnetate and Tc-99m-tartrate and the activity at the origin represents the reduced hydrolysed technetium (colloid).7
The definition of Rf is the ratio of the distance the radiochemical species travelled on the stationary phase to the distance traversed by the mobile phase’s solvent front.2 Rf may vary under different conditions but may be held constant through carefully controlled conditions.3
The most common mobile phases used for the chromatographic test include 0.9 percent saline, water, methyl ethyl ketone (MEK), acetone and methanol (Figure 3).2
Counting methods, advantages and disadvantages in purity testing for 99mTc complexes
Numerous types of instruments can be used for counting radioactivity on radiochromatography strips and some of these include radionuclide calibrator/dose calibrator, gamma counter, high performance liquid chromatography and many more. The advantages and disadvantages of some of them are mentioned below:
- Thin Layer Chromatography (TLC): with glass backing – tends to have better quality and resolution.7
- Instant Thin Layer Chromatography (ITLC): like TLC but quicker. The disadvantages of this technique are that it is much cruder – ie, no real Rf measurement but just 0-1 – and no solid support, with glass fibre or PTFE mesh impregnated with silica gel particles.7
- High Performance Liquid Chromatography (HPLC): the disadvantages are it takes up a lot of space, is expensive and very slow for radiopharmaceuticals.7
- TLC/ITLC scanner measurement (linear scanner): this method gives full profile and it is easy to analyse the sample. However, it is expensive, takes up space, has low sensitivity and it can be slow.
- Well scintillation counters: this method is limited in that if the radioactivity is increased, the count rates decrease.7 To avoid exceeding the maximum capabilities of this method, there are some methods that could be applied such as increasing the distance from source to detector, use of an attenuator on the well counter and decreasing the volume of the radioactive sample.1
- Radiogram scanners: these have the capability of counting samples exhibiting a wide range of activities and gamma cameras may be used as a well-counter having 2π geometry and an elevated background. Using a gamma camera interface with a computer, Mock and Appledorn1 have developed a daily radiopharmaceutical quality control system that provides both a tabular estimate of radiochemical purity and single image containing up to eight Tc-99m radiopharmaceuticals that can be compared with a reference image from Tc-99m radiopharmaceuticals of known purities.1
- Multiple radiopharmaceuticals: this system can be set up in a minimum amount of space and takes only one minute. This permits the sequential development and counting of chromatograms from four radiopharmaceuticals in less than five minutes. The stationary phase that can be used is Whatman 3IET paper and ITLC-SA (1-2) strips with acetone and 20 percent NaCl, respectively, to separate TcO4– and ITLC-SG (3-4) strips and water are used to separate R-Tc5 (Table 1).1
| New Class | Media/ (stationary phase) | Solvent/ (mobile phase) | Impurity | Tc-99m-kit radiopharmaceutical |
| 1-2 | 3IET | Acetone | TcO4– | Sc, MAA, DTPA, HMDP, MDP |
| 1-2 | ITLC | 20 percent NaCl | TcO4– | HIDA, PIPIDA, DISIDA |
| 3-4 | ITLC-SG | H2O | R-Tc | DTPA, MDP, HMDP, HIDA, PIPIDA |
Table 1: Multiple radiopharmaceuticals chromatographic system
Summary
99mTc-R radiopharmaceuticals are used in nuclear medicines for diagnostic and therapeutic purposes. One essential parameter in the quality control of radiopharmaceuticals is the radionuclidic/ radiochemical purity. The radionuclide must have specific nuclear characteristics to allow the achievement of good scintigraphies, to comply with the impurity’s limits specification for localisation only in the targeted organ and to be non-toxic. Various chromatographic methods are applied to determine the radiochemical purity of technetium-99m-R-labelled compounds. Currently the most common chromatographic technique used includes paper and ITLC chromatography because these techniques provide the most accurate and fastest chromatographic results. However, the counting system should be robust with the sensitivity of 1μCi,1 as there are very low activities to be detected. As a rule, 100μCi or more of Tc-99m should be applied to the chromatography strip, in order to maintain an error of less than or equal to one percent. This corresponds to a 5μl drop from a 20mCi/ml radiopharmaceutical. At this level of activity, the counting error in the determination of one percent Tc-99m TcO4– in a Tc-99m radiopharmaceutical on a paper strip developed in, for example, acetone would be approximately 100 percent of the value (1+1 percent TcO4-), assuming a sensitivity of 1μCi for the counter used.1
About the author
Anastasia Petropoulou obtained a certificate in Health and Science followed by a BSc Hons degree in Pharmaceutical Science at the University of the West of England. She has gained experience in quality control including chromatography through her degree study and by completing work in both pharmacy and the food industry. Anastasia currently works in radiopharmacy as a Radiopharmacy Technician/Scientist where she applies chromatographic techniques on radionuclides to ensure high quality, pure products are supplied to patients.
References
- Analytical Control of Radiopharmaceuticals, (1970), INTERNATIONAL ATOMIC ENERGY AGENCY, VIENA, SUBJECT GROUP: IV, Chemistry, Geology and Raw Materials/Labelled Compounds and Radiopharmaceuticals.
- Looneless SV. 2012, Quality Control of Compounded Radiopharmaceuticals, Continuing Education for Nuclear Pharmacists And Nuclear Medicine Professionals, VOLUME 15(XV), UNM COLLEGE PHARMACY.
- Sampson CB. 1999, Textbook of Radiopharmacy Theory and Practice, Third Edition, GORDON AND BREACH SCIENCE PUBLISHERS.
- Quality Assurance of Radiopharmaceuticals, 2001, Report of a Joint working party: the UK Radiopharmacy Group and the NHS Pharmaceutical Quality Control Committee Nucl Med Commun, 22: 909-916.
- Decristoforo C, Zolle I, Rakia´s F, Imre J, Hesslewood SR. 2007, Quality control methods of 99mTc pharmaceuticals. In: Zolle I (Editor), Technetium-99m pharmaceuticals. Preparation and quality control in nuclear medicine. Berlin: Springer; p 123-150.
- Seetharaman S, Ballinger JR, Sosabowski MH. 2006, Simplified Method for Determining the Radiochemical Purity of 99mTc-MAG3, Nucl. Med. Technol. vol. 34 no. 3 179-183
- Ballinger J, Blower P. 2016, Summer School in Radiopharmaceutical Quality Control Techniques, Division of Imaging Sciences King’s College London.
- https://www.ntp.co.za/resources-for-learners/
- https://en.wikipedia.org/wiki/Technetium-99m
- INTERNATIONAL atomic energy, 1992, Preparation of Kits for 99mTc Radiopharmaceuticals, IAEA-TECDOC-649, IAEA, Viena.
- J Nucl Med May 1, 2004 45 no. 5 885-891.
Issue
Related topics
Analytical techniques, Chromatography, Drug Safety, Impurities, QA/QC, Research & Development (R&D)









