Formulation confidence with recombinant albumin: the benefits of a versatile stabiliser for challenging formulations
Posted: 20 December 2019 | Dr Phil Morton (Albumedix) | No comments yet
The path to formulation of pharmaceuticals is not always straightforward, especially as products are becoming increasingly complex. Recognising strategies with the greatest advantages and considering all the options available to get the best drugs to market can greatly facilitate drug development and formulation. This article will demonstrate how recombinant albumin provides a cost effective, single solution to several stability issues, and is particularly effective when used in very early development, allowing products to be stabilised quickly in a common formulation and speeding up the lengthy route to market.

The expanding field of vaccine and biotherapeutic development gives promise for improved treatments against several diseases. Despite advancements in protein engineering, many of these therapies continue to face instability challenges, both physical and chemical, which can impact dosage form efficacy and have undesirable immunogenic responses. As an industry, there is also a shift from using proteins and peptides to complex large molecules, such as antibody drug conjugates (ADCs), new viral vaccines and cell-based therapies, all presenting formulation challenges.
Standard formulation technologies and strategies that require multiple stabilisers, including the optimisation of multiple types of sugars, amino acids and detergents, often do not present the required stabilisation capabilities. For instance, in vaccine and cell-based therapies, lipids can be broken up by standard excipient approaches, while products that require high concentrations, such as antibodies, can be limited by viscosity issues. In more advanced biopharma products, aggregation and oxidation are also issues. Today’s newer drugs also face challenges around physical (fibrillation, adsorption/ depletion, unfolding, solubility/precipitation) and chemical (deamidation, truncation, hydrolysis) degradation.
The need for a versatile and effective stabiliser when working with hard-to-formulate drug candidates, such as high-concentration biotherapeutics, highly potent drugs and unstable therapeutic peptides, is clear. An excipient, such as recombinant albumin, provides a relatively simple solution to traditional formulation strategies, offering many benefits to drug formulators, including protection against surface adsorption and the prevention of oxidation and aggregation. So, what is holding back its adoption?
History of albumin
Within the drug development industry, the stabilisation properties of human serum-derived albumin are well recognised. As regulatory guidelines have driven the need for animal-free options there has been a decline in the use of serum-derived albumin. This is compounded by historical potential risks associated with Creutzfeldt–Jakob Disease (CJD) and Acquired Immune Deficiency Syndrome (AIDS). Yet albumin is effective when used to formulate complicated products.
An excipient, such as recombinant albumin, provides a relatively simple solution to traditional formulation strategies”
Human albumin is the most abundant protein in plasma, constituting 60 percent of total plasma protein. It is responsible for the maintenance of oncotic pressure, plasma pH and the distribution
of a variety of endogenous and exogenous ligands. It contains 17 disulphide bonds that provide excellent stability and resilience to environmental stress and its large number of different binding sites mean that it can interact with a range of products to stabilise them in a variety of situations. It has also been shown that albumin can protect drug, cell and vaccine products from aggregation, non-specific adsorption and oxidation, as well as improve solubility, batch-to-batch consistency and contribute to low immunogenicity.1-7
The industry is now shifting from albumin that was originally sourced from plasma in human serum to chemically-defined (recombinant) human serum albumin.6 This shift has occurred due to several advantages of recombinant albumin, which include: certainty of supply, high purity, absence of host-derived proteases, high homogeneity, the absence of animal-derived products, high free thiol content, absence of known or unknown human pathogens, batch-to-batch consistency and the presence of an established regulatory pathway.
Albumin in use
The mechanistic properties of albumin can be grouped into four categories to better understand the functions of albumin in formulations:
1. Protection
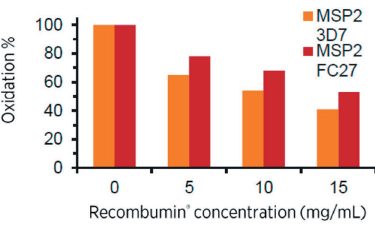

Figure 1: The degree of oxidation, after incubation in hydrogen peroxide, of malaria vaccine antigens decreases as the concentration of recombinant albumin increases.
Recombinant albumin has high antioxidation capacity stemming from the free thiol moiety at cysteine 34. This is an unpaired cysteine residue, which is present in its reduced form and can become oxidised instead of the active pharmaceutical ingredient (API) during oxidative stress.8 This protects biopharmaceuticals against modification through oxidation by scavenging oxidative free radicals when in formulation (Figure 1).
2. Coverage
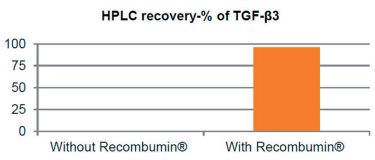

Figure 2: Prevention of drug depletion due to surface adsorption of the protein TGF-β3.
Recombinant albumin acts to prevent drug loss through non-specific adsorption due to its ability to bind to both hydrophobic and hydrophilic surfaces where it can remain attached over time. This property is utilised for low dose/high potency drugs, as the dose can be difficult to control due to its surface adsorption during production, handling and storage (Figure 2). As albumin has a predisposition to cover surfaces, it has historically been used to increase the biocompatibility of medical devices by covering their surface. Additionally, by covering these surfaces, albumin can hamper surface-induced unfolding and aggregation events of the API. Studies have shown albumin to spontaneously cover surfaces in a single protein layer, where only 1–2mg of albumin is needed to cover one square metre of surface.
3. Binding
Albumin demonstrates promiscuous binding properties, binding in either a simple 1:1 ratio or other more complex ratios, resulting in the reporting of numerous APIs binding to albumin. This ability can convey multiple effects on the formulation. For instance, by direct binding, albumin can improve solubility of otherwise difficult to solubilise molecules or prevent otherwise detrimental interactions of molecules that could cause unfolding and aggregation.6 There are also indications of albumin having the ability to bind to the exposed hydrophobic end of growing fibrils, thereby curtailing their propagation.9
4. Avoidance
Albumin has a positive second viral coefficient, where the oncotic pressure in blood is brought about by albumin repelling itself in solution. The general movement and flexibility of other entities in the solution is hampered by the albumin, which is distributed evenly in the solution. The decreased flexibility of the entities in formulation can translate into increased stability of the product.10 Although it is difficult to conceive or illustrate the effect of this during formulation, SAXS has shown that co-formulated proteins have lower flexibility when prepared with albumin without there being any direct interaction.
The cost of formulation versus long-term benefits
Often cost can be a barrier to employing a recombinant albumin strategy. Traditional formulation strategies are often cheaper, utilising simple chemicals, which should not interfere with the analysis of the product itself. Simple excipients may be satisfactory in some instances, for example, when an API is very stable and can be easily formulated, a traditional formulation strategy is likely to suffice. However, more complex molecules, such as ADCs and cell therapies, which can have a number of different stability issues, benefit from an excipient that has multiple stabilising mechanisms and, especially for cell therapies, be of a biological nature as well as very high purity and consistency when compared to simpler sugars, amino acids and detergent (SAD) excipients.

The use of a recombinant albumin can increase safety by producing a product with the correct stability profile, which in turn increases the likelihood of a product meeting regulatory requirements, saving money as drug failures have huge cost implications. Higher API processing yields can also be achieved with recombinant albumin and lower batch-to-batch variability, providing smoother and quicker development. In addition, if used early in the development cycle, it can quickly solve stability issues that would have previously delayed clinical trials and product launch, meaning the product will reach the market quicker, resulting in longer exclusivity. Recombinant albumin could also be used to further improve stability; for example, a liquid-filled pen syringe can be used as a product presentation instead of lyophilised products that need premixing before administration, potentially allowing differentiation of a product with additional cost implications.
Conclusion
For vaccine products, the inclusion of recombinant human albumin can improve stability in both liquid and solid state, as well as improving yields during harvesting and processing viruses for vaccine preparation”
The drug development industry is seeing a move away from proteins and peptides towards even more complex large molecules, such as ADCs, new viral vaccines and cell-based therapies. These therapies present greater formulation challenges and often standard formulation technologies are neither efficient, nor offer the required stabilisation capabilities. With the availability of recombinant albumin, historical risks associated with human serum albumin are eliminated, but it is still a somewhat overlooked molecule as opposed to SADs. Yet, with recombinant albumin offering so many benefits, it might be time to revisit this versatile stabiliser.
For vaccine products, the inclusion of recombinant human albumin can improve stability in both liquid and solid state, as well as enhancing yields during harvesting and processing viruses for vaccine preparation.11-14 Albumin is already used in marketed therapeutics including the M-M-R II vaccine (Merck) and the ProQuad Varicella virus vaccine (Merck).14,15 It has also been used to improve existing drugs, such as the chemotherapy drug Paclitaxel. Paclitaxel was previously formulated in solvents and other aggressive compounds, which resulted in adverse clinical events. The drug has since been reformulated using albumin and is marketed as Abraxane with significantly less adverse effects. There is so much to explore that companies may not be aware of, particularly when it comes to early development. As products become increasingly complex, the industry stands to benefit from recognising the strategies with the best advantages.
About the author
Dr Phil Morton has over 25 years’ experience in the biopharmaceutical industry within process and product development in both R&D and manufacturing environments. His experience ranges from developing and transferring purification processes to formulation development, as well as characterisation of these processes and products. Phil holds a PhD in Biochemical Engineering from Birmingham University, UK, and followed this with post-doctoral studies at Cambridge University, UK. He currently works as the Chief Technology Officer heading up Albumedix’s Technology Group.
References
- Peters T. All about albumin. San Diego, CA [etc.]: Academic Press; 2008.
- Otigari M, Giam V. ALBUMIN IN MEDICINE. [Place of publication not identified]: SPRINGER Verlag, SINGAPOR; 2018.
- Hawe A, Friess W. Stabilization of a hydrophobic recombinant cytokine by human serum albumin. Journal of Pharmaceutical Sciences [Internet]. 2007 [cited 10 December 2019];96(11):2987-2999. Available from: https://jpharmsci.org/article/S0022-3549(16)32396-6/ fulltext
- Finn T, Nunez A, Sunde M, Easterbrook-Smith S. Serum Albumin Prevents Protein Aggregation and Amyloid Formation and Retains Chaperone-like Activity in the Presence of Physiological Ligands. Journal of Biological Chemistry [Internet]. 2012 [cited 10 December 2019];287(25):21530-21540. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/ PMC3375574/
- Wang W. Instability, stabilization, and formulation of liquid protein pharmaceuticals. International Journal of Pharmaceutics [Internet]. 1999 [cited 10 December 2019];185(2):129-188. Available from: https://www.sciencedirect.com/science/article/pii/ S0378517399001520?via%3Dihub
- Tarelli E, Mire-Sluis A, Tivnann H, Bolgiano B, Crane D, Gee C et al. Recombinant Human Albumin as a Stabilizer for Biological Materials and for the Preparation of International Reference Reagents. Biologicals [Internet]. 1998 [cited 10 December 2019];26(4):331-346. Available from: https://www.sciencedirect.com/science/article/pii/ S1045105698901634?via%3Dihub
- Kamerzell T, Esfandiary R, Joshi S, Middaugh C, Volkin D. Protein–excipient interactions: Mechanisms and biophysical characterization applied to protein formulation development. Advanced Drug Delivery Reviews [Internet]. 2011 [cited 10 December 2019];63(13):1118-1159. Available from: https:// www.sciencedirect.com/science/article/abs/pii/ S0169409X11002006?via%3Dihub
- Roche M, Rondeau P, Singh N, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Letters [Internet]. 2008 [cited 10 December 2019];582(13):1783-1787. Available from: https://febs.onlinelibrary.wiley.com/ doi/full/10.1016/j.febslet.2008.04.057
- Milojevic J, Raditsis A, Melacini G. Human Serum Albumin Inhibits Aβ Fibrillization through a “Monomer- Competitor” Mechanism. Biophysical Journal [Internet]. 2009 [cited 10 December 2019];97(9):2585-2594. Available from: https://www.ncbi.nlm.nih.gov/pmc/ articles/PMC2770600/
- Sønderby P, Bukrinski J, Hebditch M, Peters G, Curtis R, Harris P. Self-Interaction of Human Serum Albumin: A Formulation Perspective. ACS Omega [Internet]. 2018 [cited 10 December 2019];3(11):16105-16117. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/ PMC6288999/
- White J, Estrada M, Flood E, Mahmood K, Dhere R, Chen D. Development of a stable liquid formulation of live attenuated influenza vaccine. Vaccine [Internet]. 2016 [cited 10 December 2019];34(32):3676-3683. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/ PMC4940209/
- Spier R. Perception of risk of vaccine adverse events: a historical perspective. Vaccine [Internet]. 2001 [cited 10 December 2019];20:S78-S84. Available from: https://www.sciencedirect.com/science/article/pii/ S0264410X01003061?via%3Dihub
- Sun W, Roland K, Curtiss III R. Developing live vaccines against plague. The Journal of Infection in Developing Countries [Internet]. 2011 [cited 10 December 2019];5(09):614-627. Available from: http://europepmc. org/article/med/21918302
- Wiedmann R, Reisinger K, Hartzel J, Malacaman E, Senders S, Giacoletti K et al. M-M-R®II manufactured using recombinant human albumin (rHA) and M-M- R®II manufactured using human serum albumin (HSA) exhibit similar safety and immunogenicity profiles when administered as a 2-dose regimen to healthy children. Vaccine [Internet]. 2015 [cited 10 December 2019];33(18):2132-2140. Available from: https://www.sciencedirect.com/science/article/pii/ S0264410X15003059?via%3Dihub
- Pipeline and Partners | Albumedix [Internet]. Albumedix. com. 2019 [cited 10 December 2019]. Available from: https://albumedix.com/partnering/pipeline/









