Tackling immune-mediated disease with CAR Tregs
Posted: 20 October 2022 | Hannah Balfour (European Pharmaceutical Review) | No comments yet
Here, Jason Fontenot, Chief Scientific Officer of Sangamo Therapeutics, discusses with EPR’s Hannah Balfour how the industry is developing chimeric antigen receptor (CAR) T regulatory cells (Tregs) as a potentially paradigm-shifting therapeutic option for conditions driven by the immune system, such as autoimmunity and transplant rejection.

In recent years there has been significant development within the cell therapy field, as chimeric antigen receptor (CAR) T-cell therapy demonstrated an ability to transform the treatment of patients with haematological malignancies. Recognising the potential of CAR T cells to act as highly targeted therapeutics, several biotechnology companies – including Sangamo Therapeutics – have established development pipelines of CAR T regulatory cells (Tregs) that aim to tackle immune‑mediated diseases, such as autoimmunity and transplant rejection.
In this article, Sangamo Therapeutics’ Chief Scientific Officer, Jason Fontenot, explains why CAR Tregs may become transformational therapeutics for immune-mediated diseases, highlighting the challenges his company has overcome to get the first ever engineered Treg therapy into clinical trials.
What are Tregs and how are they applicable to immune-mediated disease?
Jason Fontenot (JF): Tregs are a specialised subset of immune cells that have immunosuppressive properties. They can control the activity of other immune cells and are therefore able to limit inflammatory responses, such as autoimmune inflammation.
The interest in CAR Tregs is that they could be generally applicable to numerous autoimmune and inflammatory diseases. Preventing transplant rejection is one example, but other prevalent diseases such as multiple sclerosis, inflammatory bowel disease and rheumatoid arthritis could also benefit. These are all diseases that are highly prevalent in the population and are generally treated with drugs that are broadly immunosuppressive; while some of these drugs are beneficial and do ameliorate some symptoms, these patients experience side effects including increased susceptibility to infections. The promise of CAR Tregs is that they are living drugs whose effect, using the CAR technology, we can target to a specific location in the body.
For instance, in multiple sclerosis – a disease where the patient’s own immune system attacks their central nervous system (CNS) – we can localise the Tregs solely to the CNS and prevent that pathology, offering a new and improved way of treating the disease that does not require generalised immunosuppression.
Moreover, because these are living cells, we believe the effect has the potential to be durable and long-lasting. Similarly to CAR T-cell therapies used in oncology, you could treat the patient once with these cells and, because they are living, they would not metabolise like a normal drug does; instead they are able to proliferate and expand, giving a durable effect, which has the potential – at least theoretically – to be curative.
What is Sangamo working on with regard to CAR Tregs?
We are utilising Tregs that are engineered with a CAR to treat a variety of inflammatory and autoimmune diseases”
JF: We are utilising Tregs that are engineered with a CAR to treat a variety of inflammatory and autoimmune diseases. Our first product TX200, currently in Phase I clinical testing, is for renal (kidney) transplantation. We are engineering CAR Tregs to recognise a difference between the transplant recipient and the transplanted kidney itself, and therefore localise, become activated and expand in the transplanted kidney, preventing the patient’s immune system from rejecting it.
In our renal transplant programme, the expressed CAR recognises a molecule called HLA-A*02 (a human leukocyte antigen serotype) – part of a system of cell surface molecules called the major histocompatibility complex (MHC).
Every person expresses different serotypes of HLA on their cell surfaces; when we talk about getting a matched donor organ, it relates to which MHC molecules are expressed on the donor recipient’s cells versus those on the donor organ. We are taking advantage of the fact that those matches are not always perfect; the reality is that although we try to get well-matched transplants, approximately 20 to 30 percent of transplants are mismatched by at least one HLA molecule. In our current programme we are treating patients who are HLA-A*02-negative – ie, none of their cells express the HLA-A*02 molecule – but the donor kidney is HLA-A*02-positive.
We take Tregs from the patient, engineer them with a CAR that recognises HLA-A*02 and then infuse them back into the patient. In this context, the only place where HLA-A*02 will be found is on the surface of the transplanted kidney’s cells. So, when those cells start circulating, they encounter the molecule in the transplant kidney and become activated there, establishing a kind of ‘zone of immunosuppression,’ protecting that organ from rejection by the immune system. This is exciting because it offers the potential to withdraw the patient from the kind of general immunosuppression that is normally required for transplant recipients.
In the future, we are planning to go into other autoimmune diseases and have already announced two programmes – one in multiple sclerosis and the other in inflammatory bowel disease.
Supporting evidence
Fontenot highlighted that due to the early stage of the Phase I clinical trial, having only dosed the first patient in March 2022, clinical results are not yet available for TX200. He did confirm, however, that the first patient treated so far is doing well.
Meanwhile, the pre-clinical evidence in mice that provided the basis for the first-in-human study was recently published in Gene Therapy.1 The paper demonstrated the specificity, immunosuppressive function and safety of TX200 in suppressing the activity of allograft-specific effector T cells in HLA-A*02-negative recipients of HLA-A*02-positive transplants.
TX200 was found to prevent graft-versus-host disease (GvHD) in a murine model, with the cells persisting as immunosuppressive Tregs in mice for the entire duration of a three-month study. The investigators concluded that, at least in mice, TX200 is specific, stable, efficacious and safe.
How do CAR Tregs differ from T cells?
JF: There are some very interesting similarities between both the CAR T cells used in oncology and CAR Tregs under development for immunology; they are both T cells, engineered with a new CAR receptor. It is through the development of CAR-T therapies in oncology that the technology and understanding we are leveraging to produce CAR Tregs has been established, but there are some key fundamental differences.
The core distinctions are in their function and biology: effector T cells used in oncology are evolved to kill other cells that have an infection or disease; Tregs have almost the exact opposite function – they are protective cells designed to limit the effects of other immune cells.
This is important as, in the CAR T field, there have been some prominent adverse events, namely cytokine release syndrome (CRS). In CRS the amount of inflammation that occurs after the infused CAR T cells start to destroy the cancer can become toxic to the person being treated. We expect not to have this problem with Tregs, as they are anti-inflammatory and so should be much safer, just by virtue of their function.
There are lots of other very specific differences in their biology that makes manufacturing CAR Tregs unique from CAR T cells.
What challenges were presented by CAR Treg development and manufacture?
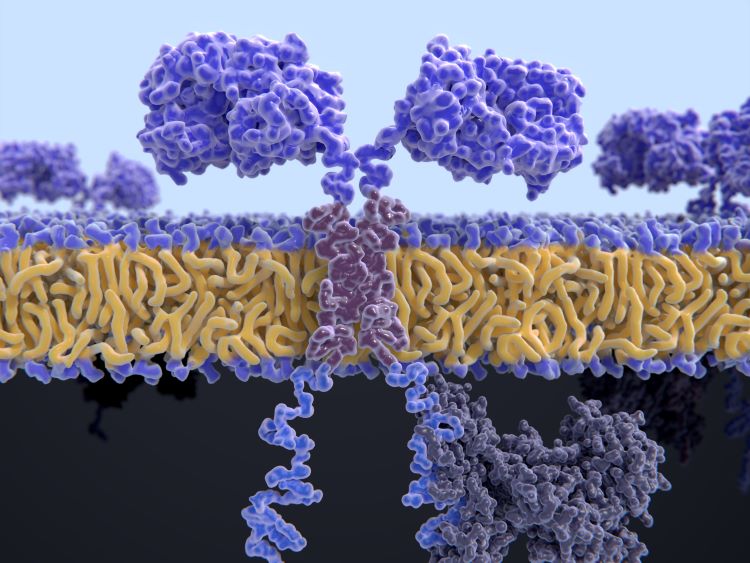

CARs are engineered cell receptors that allow T cells to recognise/attack specifically cancer cells.
JF: As a result of the differences, it has taken a lot of work to develop manufacturing processes and make sure that the Tregs have all the functionality and features that we desire. CAR Tregs are not simply a copy of an existing T-cell therapy; there are numerous peculiarities of regulatory T cells compared with effector T cells, which are both critical to their function yet also create the challenges that we had to overcome to bring this therapy to clinic.
Probably one of the most important is that Tregs only make up a very small percentage – probably less than one percent – of all your T cells. We therefore had to develop some effective and efficient tools to isolate those cells from the rest of the immune cells into a very pure population. Additionally, because it is a small population, we have to expand those cells effectively, while allowing them to retain their function and identity when engineering them to express the CAR. Both the processes of isolating and purifying a rare population and then expanding those cells to sufficient numbers in order to deliver them back to the patient required significant effort and technology development.
We have not given out details on our manufacturing process, but we believe it has a fairly similar timeline to the CAR T-cell process. I think this is reasonable as, where in the context of oncology you are often treating patients that are very sick, so there is a very limited time to deliver the therapy; in the case of autoimmune diseases, although they are devastating, they are usually not rapidly lethal and so there is a little less urgency.
Nonetheless, it is important for us to try and manufacture the product as quickly as possible because it also allows us to treat patients effectively. We are building upon all the technological development that has supported the progress of cell therapies for cancer to enable us to create and produce treatments more effectively and efficiently. This same technological base is enabling the CAR T field to increase the speed at which it can deliver drugs.
What makes cell therapy an exciting opportunity for the immunology field?
Even if they cannot be a one-time treatment, CAR Tregs could be once every four or five yearly treatments”
JF: What is so exciting about Tregs is that a) they have the potential to be very targeted to a specific area in the body, without all the side effects associated with generalised immunosuppression, and b) we really believe they have the potential to be used as a long-lasting one-time treatment.
The potential for them to be curative therapies is a fundamental transformation in the way that we treat these diseases. Patients with multiple sclerosis or rheumatoid arthritis, for example, are currently treated with therapies, either antibodies or small molecules, for the rest of their lives. While these can be effective, they are associated with significant side effects.
Even if they cannot be a one-time treatment, CAR Tregs could be once every four or five yearly treatments, so it is fundamentally a very different approach to treating these diseases.
It is early days for these therapies; as far as we know, Sangamo is the first company to test an engineered Treg in humans. I think the excitement around CAR Tregs, and the number of companies that are also trying to develop them, shows the thrilling potential of these drugs. I have been working in this field for a long time and am incredibly excited to see what the future holds.
Note: Other biotechs currently developing CAR Tregs include Kyverna Therapeutics, Sonoma Biotherapeutics, Abata Therapeutics, GentiBio and Quell Therapeutics.
About the author


Hannah Balfour is the former Science Writer for European Pharmaceutical Review.
Reference
- Proics E, David M, Mojibian M, et al. Preclinical assessment of antigen-specific chimeric antigen receptor regulatory T cells for use in solid organ transplantation. Gene Therapy. 5 Aug 2022. DOI: 10.1038/s41434-022-00358-x.
Issue
Related topics
Related organisations
Related drugs
Related people
Related diseases & conditions
Inflammatory Bowel Disease, Multiple Sclerosis (MS), Rheumatoid arthritis (RA)









