Computed tomography for in-depth investigation of freeze-dried products
Posted: 1 March 2023 | Henning Gieseler (FAU Erlangen-Nürnberg), Teresa Siegert (FAU Erlangen-Nürnberg) | No comments yet
In this article, Teresa Siegert and Henning Gieseler, discuss the benefits of applying computed tomography in the production of freeze-dried pharmaceutical products, as well as the hurdles that must be navigated to realise its potential.


Three-dimensional images using computed tomography (CT) provide a valuable, non‑invasive insight into the inner morphology of freeze-dried products. This technology allows the pore structure to be visualised in a similar way to scanning electron microscopy. It enables the detection and traceability of structural alterations and/or impurities as well as verification of the container closure integrity. However, challenges with the technique such as long measurement time to assure sufficient resolution and quality losses from the non-invasive analysis through the glass vial must still be overcome.
Importance of CT in pharmacy
Industrial CT is an established method for reliable product and process development, process monitoring and requalification. While traditional optical methods can only assess the surface, X-ray technology can be used to display the internal structure, and thus may detect defects and deviations in geometry. For this reason, CT has already been used for several years in many branches of industry as a non‑destructive method to better characterise manufactured components or products.
X-ray technology is also increasingly used in pharmaceutical R&D, particularly for examining solid dosage forms, an application for which this technology can be seamlessly adopted. Creating a profile of the different densities within a tablet enables examination of its inner structure. These differences in structure are a result of the tablet press and thus provide insight into the process quality.1 In addition, the CT images reveal the distribution of the active ingredients and help to account for fracture behaviour. Impurities can also be detected, as they usually have a higher density than the tablet ingredients.2,3
Current CT applications in freeze drying
Computed tomography is also used in the field of freeze drying. By visualising the internal pore structure, the effects of different freezing conditions and/or situations can be investigated.4 The freezing rate influences the pore size and distribution, which is then detected in the 3D image, allowing the process parameters to be optimally adapted to the desired properties of the end product. Furthermore, it can be used to estimate the mass transfer rate during primary drying.5
Possible future turnkey role for CT in freeze drying
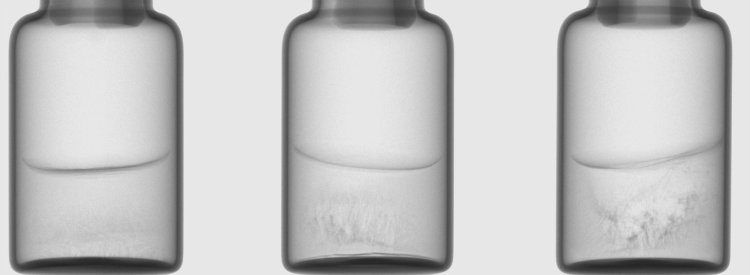

Figure 1: X-ray images of vials with a solution of S-adenosyl-L-methionine (100 mg/mL) showing different degrees of cake microcollapse. Images taken in the CTportable 25.50 micro tomograph (Fraunhofer IIS, Fürth, Germany).
There is, however, a far greater, but often overlooked, potential application for CT in pharmaceutical freeze drying. Even with basic two-dimensional (2D) X-ray images, freeze dried cake structures can be detected. While an elegant cake appears homogeneous with uniform colouration, if microcollapse occurs during drying, the highly porous material starts to shrink and a more heterogeneous structure is formed – these areas then appear darker in the X-ray image. Thus, it is possible to detect structural imperfections that are not visible from the surface and to adjust the drying conditions accordingly (Figure 1). If three-dimensional (3D) reconstructions are captured from the cakes, these defects can be more clearly identified. The honeycomb-like pore structure, which can be seen in elegant cakes, fades into an irregular network with rod-like structures, which are partially collapsed and have drop-shaped ends. Using this technology, even the smallest indications of microcollapse can be detected without an invasive examination using scanning electron microscopy.6
In a similar way to tablets, an inspection for impurities could also be carried out in freeze-dried products. Due to the low solid content in the freeze-dried cake and the associated low density, the cake distinguishes well from the impurities (Figure 2). These contaminations are sometimes carried into the vial during the manufacturing process, for example, particles of silicone or metal can be introduced via the stopper. If glass breakage occurs, there may also be contamination by small glass particles. Since these particles are usually very small and are located inside the cake, they cannot be detected during conventional optical inspection, thus posing great potential risk to patients. An automated 100 percent inspection using X-ray technology could detect and reject contaminated vials before entering the market. If certain contaminations occur more frequently and thus indicate a defective device, faulty process steps can be detected and prevented at the earliest possible stage.
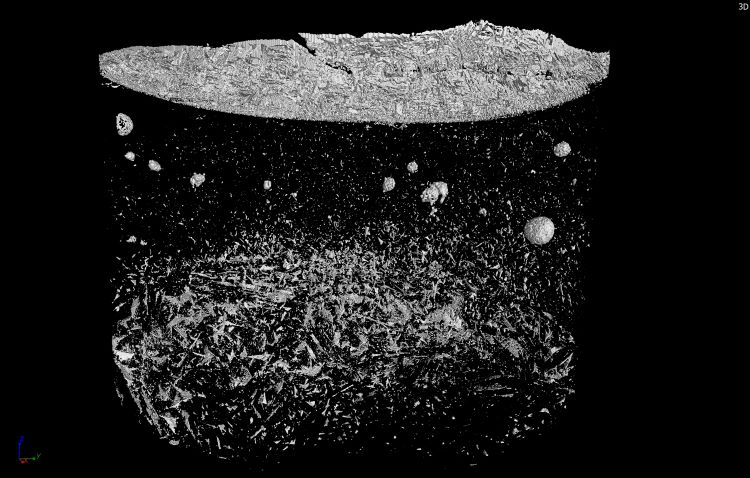

Figure 2: 3D reconstruction of a vial with 50 mg/mL mannitol formulation and added ethylene-propylene-diene rubber impurities. The impurities as well as a heterogeneous structure of the cake and the formed skin on top can be seen. Recorded and reconstructed by C. Schulbert (Geozentrum Nordbayern, Erlangen, Germany) in the Phoenix V|tome|x s (Waygate Technology, Hürth, Germany).
Optical coherence tomography freeze‑drying microscopy (OCT-FDM)7 could also potentially be used based on computed tomography, thus enabling the structural changes within the cake during drying to be observed and displayed in 3D. This provides information about the behaviour of the formulation during freeze drying in a realistic measurement environment. The collapse temperature determined by this technique is also closer to the real product behaviour than, for example, with a DSC measurement.7 These insights can be used to optimise the drying parameters.
Another possible application of CT is verifying the sealing of vials following closure with aluminium caps. To ensure sterility, the vial must be properly sealed. A faulty closure of the aluminium caps could be detected by the X-ray images from density differences of the vial, stopper and cap. If, for example, the aluminium cap sits crookedly on the stopper or does not enclose the vial rim properly, this will be detected in the CT.
…Computed tomography (CT) could enable full automated characterisation of vials in the future.”
The aforementioned examples illustrate that CT could enable full automated characterisation of vials in the future. Since traditional visual inspection can already be replaced by automated camera systems,8 CT could lead to reduction of failure by human inspection, cost savings and higher quality of freeze-dried products.
Challenges in freeze drying applications
Before CT can be applied in industrial freeze drying, several challenges must be overcome. First, desirable resolution is still an issue. Accurate visualisation of the internal pore structure requires a sufficiently small pixel size. However, with good resolution, the time parameter becomes crucial. The better the resolution, the longer the measurement will take. For example, a high resolution measurement in, for example, the SkyScan 1275 micro tomograph (Bruker MicroCT, Kontich, Belgium) with a pixel size of 18µm and a rotation step of 0.25° takes about one and a half hours.6
Before CT can be applied in industrial freeze drying, several challenges must be overcome. First, desirable resolution is still an issue. Accurate visualisation of the internal pore structure requires a sufficiently small pixel size. However, with good resolution, the time parameter becomes crucial. The better the resolution, the longer the measurement will take.”
A measurement time in the range of several minutes can achieve a good resolution. While this is quite feasible in a research environment, it is not applicable on an industrial scale. On average, 30,000 – 60,000 vials are conventionally dried in a commercial batch over a period of approximately three days. Subsequently, a 100 percent inspection of 60,000 vials with just one minute measurement time would take around 42 days for final product release. If the duration of the scan is further reduced by increasing the pixel size and the rotation speed of the sample, there is a significant loss of resolution. So-called in vivo-CT devices could offer a solution here, as they are designed to perform rapid scans of animals and thus shorten the measurement time to the range of seconds.9
If CT is used to detect contaminants, resolution is even more critical, as particles can reach diameters of less than 50µm. The detectability of the particles is not only limited by the technical capabilities of devices; physical phenomena also influence the detectability. Since X-rays are electromagnetic radiation, they can interact with matter in different ways. This causes the X-ray beam to attenuate as it passes through matter, the degree to which is dependent on the type of material. The intensity thus decreases exponentially with the length penetrated.10 This poses a major problem for particle detection in freeze-dried cakes, as the diameter of the vial is significantly larger compared to the particle size. Thus, the density difference between cake material and particle is negligible for attenuation. This means that the particle can no longer be detected. Increasing the excitation voltage would produce better penetrability of the material, but the image contrast would be reduced.11,12


Figure 3: X-ray images of a molded glass 20mL vial (left) and a tubular glass 20mL vial (right). Images taken in the CTportable 25.50 micro tomograph (Fraunhofer IIS, Fürth, Germany).
The influence of the glass vial on the CT images must also be accounted for. Compared to the cake matrix, glass has a significantly higher density and thus absorbs X-rays very well. The image contrast is therefore lower than in an analysis without a vial,6 but this would not constitute a non-invasive method. The type of glass must also be considered. Tubular glass shows a homogeneous wall thickness, whereas molded glass sometimes shows differences in wall thickness. As a result, the glass appears uneven and darker in the X-ray image, making it harder to see pores or impurities (Figure 3).
Conclusion
As technology continues to evolve and given that CT is an established technique, its importance in freeze drying will likely increase. Its numerous possible applications illustrate great potential to improve the quality of freeze-dried cakes. As a result, rejected product vials can be reduced and the safety of the application is guaranteed. Further work is needed to determine whether the current hurdles can be overcome and whether CT can be used as an inspection device in the freeze‑drying industry in the future.
About the authors




Henning Gieseler, PhD is a licensed pharmacist in Germany, he holds a PhD in pharmaceutics and received the venia legendi in pharmaceutical sciences in 2010 from the Friedrich-Alexander-University Erlangen-Nuernberg with the thesis “Quality by Design (QbD) in Freeze-Drying Using Advanced Process Analytical Technology”. He currently works as a Chief Scientific Officer at GILYOS GmbH, a CRO for freeze-dried products, which he founded in 2010. In addition, he serves as adjunct faculty at the FAU Erlangen-Nuernberg and is group leader of the Freeze Drying Focus Group (FDFG) at the Department of Pharmaceutics in Erlangen.
References
1. Sinka IC, Burch SF, Tweed JH, Cunningham JC. Measurement of density variations in tablets using X-ray computed tomography. Int J Pharm 2004; 271(1-2): 215–24 [https://doi.org/10.1016/j.ijpharm.2003.11.022][PMID: 15129988]
2. Hancock BC, Mullarney MP. X-ray Microtomography of Solid Dosage Forms. PharmTech 2005 Apr 1.
3. Sovány T, Kása P, Vakli K, Pintye-Hódi K. X-ray computed microtomography for determination of relationships between structure and breaking of scored tablets. X-Ray Spectrom. 2009; 38(6): 505–9 [https://doi.org/10.1002/xrs.1205]
4. Mousavi R, Miri T, Cox PW, Fryer PJ. A Novel Technique for Ice Crystal Visualization in Frozen Solids Using X-Ray Micro-Computed Tomography. J Food Science 2005; 70(7): e437-e442 [https://doi.org/10.1111/j.1365-2621.2005.tb11473.x]
5. Foerst P, Melo de Carvalho T, Lechner M, et al. Estimation of mass transfer rate and primary drying times during freeze-drying of frozen maltodextrin solutions based on x-ray μ-computed tomography measurements of pore size distributions. J. Food Eng. 2019; 260: 50–7 [https://doi.org/10.1016/j.jfoodeng.2019.05.002]
6. Haeuser C, Goldbach P, Huwyler J, et al. Imaging Techniques to Characterize Cake Appearance of Freeze-Dried Products. J Pharm Sci 2018; 107(11): 2810–22 [https://doi.org/10.1016/j.xphs.2018.06.025][PMID: 30005985]
7. Mujat M, Greco K, Galbally-Kinney KL, et al. Optical coherence tomography-based freeze-drying microscopy. Biomed Opt Express 2012; 3(1): 55–63 [https://doi.org/10.1364/BOE.3.000055][PMID: 22254168]
8. ATS Automation Tooling Systems. Software & Inspektion; 2021 [cited 2022May] Available from: URL: https://atslifescienceseurope.atsautomation.com/technologien/softwareloesungen/#1618856837892-aa8180ec-511b.
9. In Vivo MicroCT | High Resolution | Desktop; 2022 [cited 2022Apr] Available from: URL:
https://www.bruker.com/en/products-and-solutions/preclinical-imaging/micro-ct/skyscan-1276.html.
10. Ardenne MB. Elektronen-Übermikroskopie: Physik · Technik · Ergebnisse. Berlin, Heidelberg: Springer Berlin Heidelberg 1940.
11. Turner JE. Atoms, Radiation, and Radiation Protection. 3rd completely rev. and enl. ed. Weinheim: Wiley 2007.
12. Krieger H. Grundlagen der Strahlungsphysik und des Strahlenschutzes. 4. Aufl. 2012. überarb. u. erw. Wiesbaden: Vieweg+Teubner Verlag 2012.
Issue
Related topics
Analytical techniques, Biopharmaceuticals, Production, Technology









