siRNA drug delivery across the blood-brain barrier in Alzheimer’s
Posted: 24 October 2023 | Carrie Taylor (BOC Sciences) | No comments yet
Carrie Taylor, Director of Research & Development at BOC Sciences examines the key pathologies in Alzheimer’s and highlights several promising siRNA drug delivery systems that offer potential in helping to overcome the neurodegenerative disease.

Alzheimer’s disease (AD) is a very common neurodegenerative disease in older people, which mainly affects the patient’s cognition, memory and independence. However, it also leads to a decline in the quality of life and the condition can be fatal. According to a report by Alzheimer’s Disease International, the number of dementia patients worldwide is expected to reach 152 million in 2050, of which about 60-70 percent will be AD patients.1
Drugs that have been approved for marketing regarding AD can only moderately relieve patients’ symptoms and have some adverse effects. Therefore, the development of a drug that has a safety profile and can target the underlying causes of AD pathology to achieve a cure for AD has been an ongoing pursuit of researchers in this field. Currently there have been recent advances in the study of AD pathology, and new strategies for the treatment of siRNA drug delivery.
Several pathologies play a key role in Alzheimer’s, including:
Tau Protein Clearance Disorder
Tau is a microtubule-associated protein that plays a crucial role in maintaining the microtubule network of axons and protecting axons from enzymatic degradation. One of the pathological causes of AD is the presence of 4R isoforms of Tau protein, which become misfolded and excessively phosphorylated. These phosphorylated Tau proteins (p-Tau) aggregate into oligomers, leading to the formation of neurofibrillary tangles and eventual neuronal cell death. Additionally, p-Tau can spread to neighbouring neurons and glial cells through a prion-like mechanism, facilitating the propagation of misfolded Tau protein in the brain of AD patients and ultimately contributing to brain atrophy.
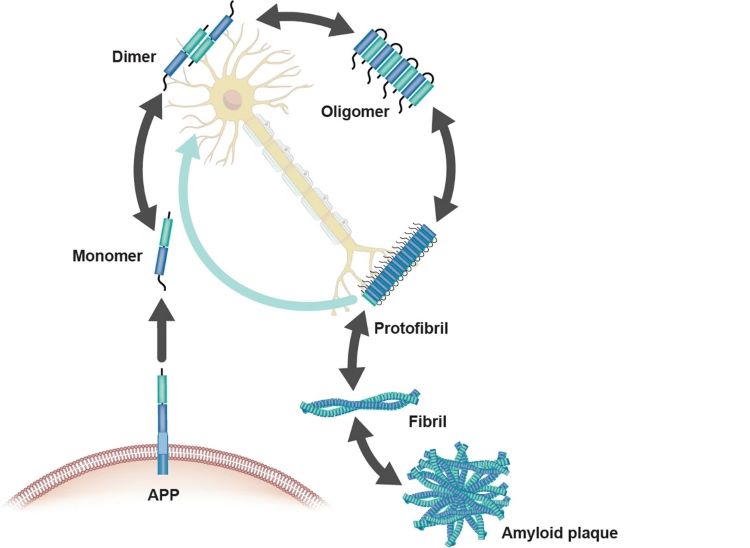
Amyloid β-(Aβ) aggregation
One of the symptoms in AD patients is the deposition of Aβ protein, which is also widely recognised. During the generation of amyloid, amyloid precursor protein (APP) undergoes enzymatic cleavage to form monomers of Aβ. In a healthy brain, Aβ primarily exists in a soluble form and is metabolized by β and γ-secretases. However, in the pathological state of AD, Aβ metabolism is disrupted, leading to its accumulation in the neocortical regions of the brain, where it forms peptides of varying sizes. These peptide oligomers aggregate at synapses to form amyloid plaques, inhibiting neuronal transmission and ultimately leading to neuronal death.
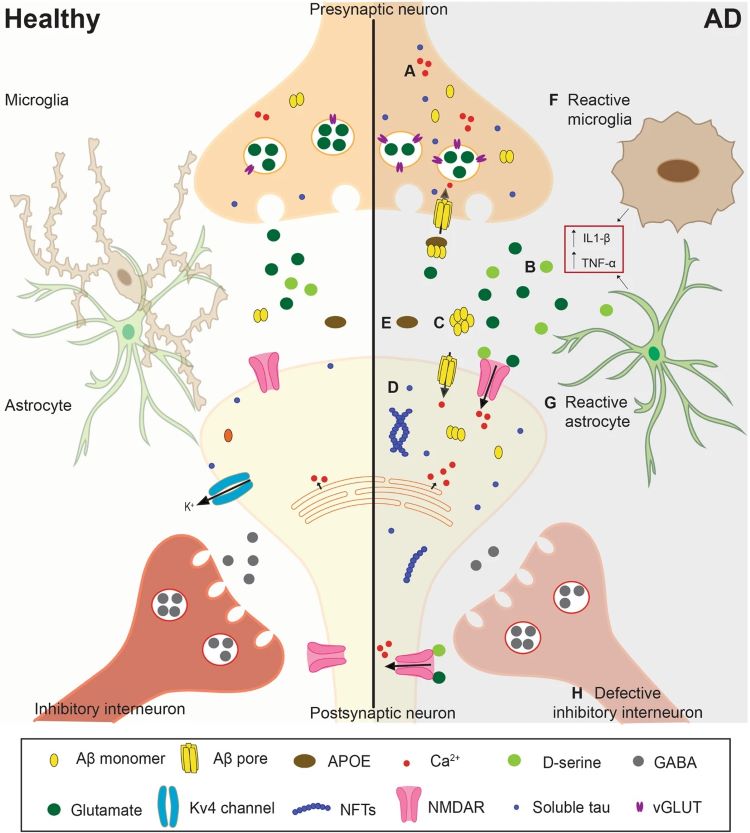
Neuroexcitotoxicity and reduced synaptic plasticity
The aggregation of Aβ and Tau proteins promotes the occurrence of excitotoxicity in neurons and reduces synaptic plasticity mediated by NMDA and AMPA receptors (AMPARs). In the state of AD, neuroinflammation triggers the presynaptic release of glutamate, which binds to NMDA and AMPA receptors in postsynaptic neurons, causing excessive calcium influx, leading to excitotoxicity and the generation of reactive oxygen species (ROS).
Furthermore, Aβ oligomers promote excitotoxicity by disrupting glutamatergic synapses and plasticity, explaining the characteristic cognitive deficits observed in AD. Aβ-dependent synaptic toxicity leads to the dephosphorylation of AMPARs, facilitating their removal from postsynaptic sites. The reduction in AMPAR-mediated currents indirectly contributes to depressive symptoms in patients, and depression is associated with dendritic spine degeneration and memory loss.
Mitochondrial dysfunction
In characteristic pathological conditions of AD patients, local hypometabolism or impaired glucose tolerance occurs due to mitochondrial dysfunction. Additionally, neuronal mitochondria undergo oxidative stress, ROS that damage DNA, lipids, and proteins. Furthermore, Aβ aggregates dock onto sodium pumps, inhibiting vasodilation through the ROS-PKC pathway in the brain, reducing blood flow to the brain, exacerbating AD.
Unbalanced Ca2+ concentration
Ca2+ plays a role in mitochondrial metabolism and mitochondrial membrane depolarization. In the brains of AD patients, Ca2+ concentrations are higher than in healthy individuals. Furthermore, the increase in calcium ion concentration leads to excessive stimulation of neurons by glutamate, activating nitric oxide synthase, resulting in an overproduction of NO that damages the mitochondrial membrane. Additionally, the substantial influx of Ca2+ into mitochondria reduces ATP production. Due to the elevated calcium ion levels, the endoplasmic reticulum is unable to properly process protein structures, leading to misfolding, accumulation, and aggregation of proteins.
siRNA therapy in Alzheimer’s
“delivery of siRNA for AD therapy via intravenous or intramuscular routes is a viable strategy. However… various intracellular and extracellular barriers [must be overcome] to reach its target site, such as the blood-brain barrier (BBB)”
siRNA is a non-coding RNA molecule with a length of no more than 20-24 nucleotides. It can impact the transcription of genes involved in the pathogenesis of AD, leading to gene silencing. Depending on patient compliance and cost-effectiveness, the delivery of siRNA for AD therapy via intravenous or intramuscular routes is a viable strategy. However, the delivery of siRNA must overcome various intracellular and extracellular barriers to reach its target site, such as the blood-brain barrier (BBB). Researchers are looking for ways to optimise nanomaterials for nucleic acid-based AD therapies.
siRNA delivery systems
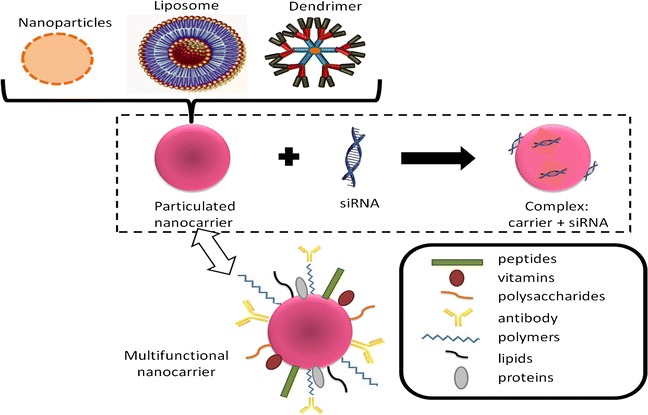
There have been reports of various nanoparticles being used as siRNA delivery methods. For example, nanoparticles such as polyethylene glycol-coated nanoparticles, magnetic nanoparticles, solid lipid nanoparticles, peptide-conjugated nanoparticles, virus glycoprotein-conjugated nanoparticles, and micelle nanoparticles are gradually being developed.
dendritic polymers are being studied as critical delivery systems for treating neurodegenerative diseases and have been proven effective in delivering intact siRNA to the desired target sites”
In addition to nanoparticles, lipid-based formulations with both hydrophilic and hydrophobic properties, high bioavailability, biodegradability, and enhanced precision are indispensable for packaging active drug components like siRNA in therapeutic applications within this field.
Furthermore, dendritic polymers are being studied as critical delivery systems for treating neurodegenerative diseases and have been proven effective in delivering intact siRNA to the desired target sites.
Target genes for siRNA in AD
Decades of research have unveiled multiple genes associated with the onset and pathogenesis of AD. These genes can be further categorised into protein-coding sequences (genetic sequences that produce proteins) and non-protein sequences (genes that regulate the function of protein-coding sequences but do not produce proteins themselves).
Protein-coding sequences
APP gene
The APP gene encodes a transmembrane protein known as amyloid precursor protein. Enzymatic cleavage of APP leads to the formation of toxic Aβ aggregates, which create plaques in the brain that impair cognitive function. Multiple mutations in APP are believed to be one of the causes of AD.
Apolipoprotein E (APOE) gene
In the brain, the APOE gene is primarily expressed in astrocytes and microglial cells. Activation of the APOE gene stimulates the transcription of APP, which, when cleaved, generates Aβ aggregates. Additionally, APOE can trigger neuroinflammation, exacerbating amyloid and Tau pathology. Using siRNA to inhibit APOE, particularly the APOE ε4 allele, can significantly improve AD pathology.
PSEN1 gene
The PSEN1 gene encodes a subunit of γ-secretase, which is responsible for cleaving Aβ from APP, leading to the aggregation of amyloid in the brain. Several gene mutations have been identified and targeted to alleviate symptoms associated with the PSEN1 gene in AD.
Microglia-related genes
Activation of microglial cells exacerbates Tau pathology and leads to the secretion of inflammatory factors. These cells can directly damage neurons or activate neurotoxic astrocytes. Several genes are associated with the activity of microglial cells in AD and are referred to as microglia-related genes. For example, cell surface protein TREM2, widely expressed in brain microglial cells.
New genes
As research progresses, in addition to the classic gene targets mentioned above, many new gene targets have been discovered, advancing the field of AD therapy. Examples include the SORL1 gene, which encodes Sortilin-related receptor and the PLA2G4E gene, which can restore spatial memory deficits.
Non-protein coding sequences
In addition to protein-coding sequences, non-protein coding sequences such as miRNA are also believed to play unique roles in the progression of AD. For example, three miRNAs—miR-146, miR-21, and miR-155—have been found to regulate the release of cytokines and may be synthesised and used as potential siRNA in the treatment of inflammation-induced neuronal damage by astrocytes in AD patients.
siRNA delivery systems targeting selected genes for AD therapy
Silencing [specific] genes using appropriate siRNA delivery systems could lead to significant breakthroughs in AD treatment”
Silencing these genes using appropriate siRNA delivery systems could lead to significant breakthroughs in AD treatment. Some researchers have developed siRNA delivery treatment strategies targeting genes such as APP, BACE-1, APOE, and PSEN1. These methods not only address inherent issues like short half-life and poor membrane permeability of siRNA but also target the pathological states characteristic of AD, significantly reducing the formation of amyloid plaques.
Another siRNA approach for neuronal regeneration has opened up new avenues for AD treatment. To address the progressive degeneration of cholinergic neurons in the cortical regions of the brain, a recently developed complex composed of PEG-PEI/ROCK-II siRNA induces gene silencing of ROCK-II mRNA and subsequently promotes axonal regeneration.
Ongoing research into the pathology of AD is progressing, and siRNA therapy, as a promising approach, needs to address issues such as short half-life, renal excretion, reticuloendothelial system capture, endosomal entrapment, and off-target effects. With more research conducted by biopharmaceutical companies and the academic community, cost-effective and simplified solutions are expected to emerge, producing more siRNA-based treatment approaches in the near future.
About the author
Carrie Taylor is Director of Research & Development of BOC Sciences.
References
- Numbers of people with dementia worldwide. [Internet] Alzheimer’s Disease international. 2020. [cited 2023Oct]. Available from:
https://www.alzint.org/about/dementia-facts-figures/dementia-statistics/
Related topics
Biopharmaceuticals, Clinical Development, Drug Delivery Systems, Drug Development, Drug Safety, siRNA, Technology, Therapeutics