Maintaining vaccine security
Posted: 20 July 2006 | | No comments yet
Ninety per cent of the world’s medical research funding is being spent on just ten per cent of the world’s health problems, mostly those afflicting the residents of wealthy countries.
Vaccines are the poor relative of the $300 billion pharmaceutical industry with a market share of $6 billion (2002 WHO). As a result, many manufacturers are leaving the vaccine market.
Ironically, recent concerns in the West over the possibility of pandemic diseases such as bird flu or that of bioterrorist attack has highlighted the need for improved vaccines that can be stockpiled to enable quick response and for improved production methods so that output can be scaled up to meet demand. Many of the world’s foremost vaccine companies are now engaged in bio-defence activities.
The need for global action on vaccines for the developing world has also been given momentum by the Bill and Melinda Gates Foundation. The Foundation’s Grand Challenge for Global Health is awarding grants of $450 million to encourage research into a range of health priorities.
It might be that this Cinderella industry is about to receive a royal reception.
Market demand
Nowhere is the impact of ability to pay on market supply more acute than within the vaccine industry. Of the 1500 new medicines approved in the last 25 years, only 20 have been related to the diseases of the developing world (Gates Foundation). Of the global vaccine market only 18 per cent goes to developing countries (GAVI)
The problem is that many of the conditions that affect the four billion people that live in poverty don’t exist in the Western world and this is reflected by a lack of investment in this research.
Until recently the vaccines used in the developing countries were the same as those in the industrialised countries.
Difficulties in predicting demand meant that there was over-production of the common childhood vaccines. These were then bought at relatively low cost for use in developing countries.
The change came with the development of newer, more expensive vaccines. Manufacturers have focused on these high profit vaccines, with the result that there are shortages of the cheaper vaccines for the developing world.
The problem is two-fold; firstly new vaccines for the developing world need to be based on the forms of bacteria that circulate in developing countries and, secondly, new vaccine substitutions for the developed world must meet increased regulatory requirements, such as the removal of mercury-based thiomersal which has been widely used as a preservative. The result in the West is a switch to the more expensive single-dose vaccine vials, which places greater demands on manufacturing capacity and has undermined the practise of tiered pricing which makes excess vaccine available to the developing world at a lower price.
The global vaccine market is also becoming increasingly diversified not only in the type of antigens being supplied but also the number of vaccines being provided for national immunisation programmes.
The US immunisation recommendations for infants and adults include 13 antigens; the WHO recommends nine. Also in the US and Europe a single dose thiomersal-free vials are mandated whereas vaccine supply to the developing world depends on multidose vials with thiomersal as a preservative.
Market supply
A series of mergers between major pharmaceutical companies coupled with a shrinking manufacturing base for low-profit vaccines has resulted in a global shortage of some vaccines.
However there are signs that this will be addressed. While new vaccine development is carried out mainly by large multinationals there is increasing capacity for manufacturing emerging in the developing countries, most notably in India and Pakistan. It is expected that they will play an increasing role in product development and they have access to large home markets.
Several developing country manufacturers have entered joint agreements with major vaccine manufacturers for the production of some vaccines, particularly GlaxoSmithKline, which has agreements with companies in Brazil, Egypt and Cuba and a network of manufacturers is emerging to develop strategies for the production of high quality vaccines.
In 2000 50 per cent of UNICEF’s vaccine procurement was purchased from ‘emerging producers’, so they already have a major influence on the global supply picture.
Problems with current vaccines
It is estimated that more than two million children die from vaccine preventable disease each year1. A major contributing factor to this problem is the difficulty in getting potent vaccines to remote areas. Similarly, many injectable pharmaceuticals such as insulin require storage in refrigerators and are difficult to deliver to remote and developing areas of the world.
There is a major need that vaccines and drugs should be thermally stable to both heat and freezing and ready-to-inject with no preparation in the field.
For more than 100 years most vaccines have been in the form of unstable liquids that require constant refrigeration from the time of manufacture to their injection in the field. This ‘cold chain’ costs more than $200 million p.a.2 and often breaks down. Contributing factors will include poor logistical infrastructures, intermittent power supplies and lack of basic refrigeration facilities.
The installation of a cold chain itself has introduced another problem i.e. the vulnerability of most vaccines to freeze damage caused by cold packs that are not tempered to 0°C after removal from the freezer and before use in the insulated boxes used for final transport. Up to 70 per cent of vaccines used by national infant outreach immunisation programmes can be lost from freezing alone3.
Lyophilisation of vaccines, such as freeze-dried attenuated measles virus, is a widely used technique for production of dried formulations. Whichever method is used, the dried formulations are stable for many years but require constant refrigeration and are only stable at room temperature for a few hours after reconstitution. Furthermore there are frequent tragic mistakes in reconstitution with contaminated or incorrect diluents4. As a result, staff training is required for administration of such vaccines and consequently costs rise. The need for reconstitution creates an extra burden on time, which reduces the efficiency of large vaccination programmes.
A final consideration of vaccination programmes is one of compliance. In most cases vaccines require multiple booster injections spaced over a period of time in order to generate a fully protective response in the recipient. Not only does this increase the cost of raw materials required but the cost of administration of those vaccines is doubled or trebled. However the major problem arising is that many patients do not return for the booster vaccination. Compliance rates are not precisely known but it is widely accepted that a substantial proportion of patients do not receive their full complement of injections.
It is therefore clear that future generations of vaccine will need to be stable at elevated temperatures, ready to administer without need for handling and without a requirement for booster shots.
Ways forward
In order to address these problems Cambridge Biostability Ltd (CBL) has developed stable, dry formulations of vaccines and drugs that contain the actives stabilised in water-soluble glass.
One generic process consists of stabilised actives in glass microspheres which are suspended in biocompatible anhydrous liquids (Stable Liquid Vaccines). The latest discovery is a method for vaccine stabilisation which may remove the need for expensive booster vaccinations – Controlled Release. Formulations of stable vaccines containing calcium phosphate have been shown to gently stimulate the recipient’s immune system during the course of several months. With this continuous dosing there is no need for the two or more booster shots commonly required.
Stable liquid vaccines
The formulation technology developed by CBL consists of stabilising the actives in water-soluble glass microspheres which are suspended in biocompatible and density-matched anhydrous liquids in which they do not dissolve, thus forming two-phase liquids (Figure 1). When injected, the glass microspheres dissolve in body water releasing potent vaccines or drugs and the anhydrous liquids are either exhaled in the breath or rapidly metabolised. These stable liquid formulations can be stored at high ambient temperature for at least three years, are completely resistant to freeze damage and can be injected without any preparation at the point of use. Due to the mechanism of stabilisation, the product – which is in solid solution in the dry glass – is stable up to the softening point (glass transition temperature, Tg) of the glass. This is true even of products which are extremely unstable in aqueous solution in their native form. Recent formulations produced at CBL using this technology have been demonstrated to have a Tg higher than 70°C.


These products promise to radically improve the way vaccinations and therapeutic drugs are delivered worldwide.
The anhydrous liquids used in these preparations are of two types: high density fluorocarbon liquids and low density metabolisable oils. In order to produce physically stable suspensions that do not separate into two phases, the formulations must contain density-matched high density or low density glass microsphere particles. Crucial to this technology was the development of a process to manufacture large quantities of highly polished spherical glass micro-particles of known density. Although emulsion drying, freeze drying and spray-freeze drying were all investigated, spray drying was finally chosen as the process of choice.
CBL has developed processes where precise control of density of the powders may be achieved. To increase density is relatively easy; addition of a dense but inert excipient increases the overall density of the powder. Reduction of density is achieved by a process whereby precisely calibrated volumes of biocompatible gases are generated and trapped within the spherical particles, yielding particles of predetermined low density, ‘aerospheres’.
These suspensions of glass microspheres are indefinitely stable on storage; the particles have neutral buoyancy and neither settle nor float (Figure 2). This enables great simplification of the manufacturing method as the particles do not need to be particularly small in order to stay in suspension. The highly polished glassy spherical particles roll past each other with an inherent lubricity, enabling very high solids contents up to 80% to be achieved in viscous creams. Preferred concentrations of 5-35% yield highly mobile, water-like injectable liquids. As the labile active ingredients are embedded in glass particles and the carrier liquids used are dry there is no degradation due to light, heat or oxygen.
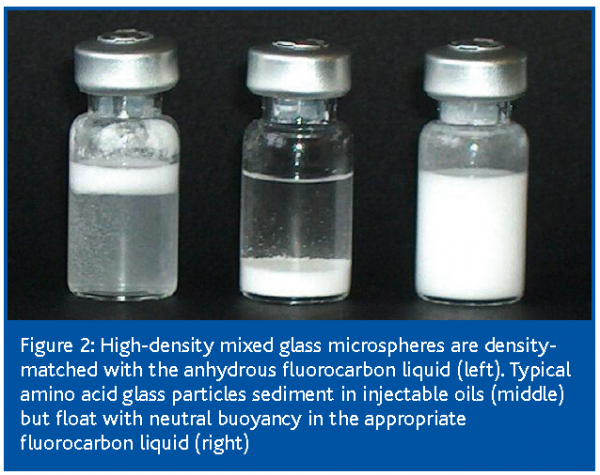

Relating manufacture to demand
Currently vaccines are made through batch production which means that scaling up production quickly is problematic. CBL’s use of spray drying addresses this issue.
In order to manufacture stable liquid vaccines (SLV) on a commercial scale a specialised manufacturing plant has been developed for Phase I clinical trial material. It is proposed that this facility will be used to develop and validate a new manufacturing process. A schematic representation of the facility is illustrated in Figure 3.
A new aseptic spray dryer (ASD1) has been developed in association with GEA Niro A/S (Copenhagen, Denmark) and will be operated under contract by Nova Laboratories (Leicester, UK).
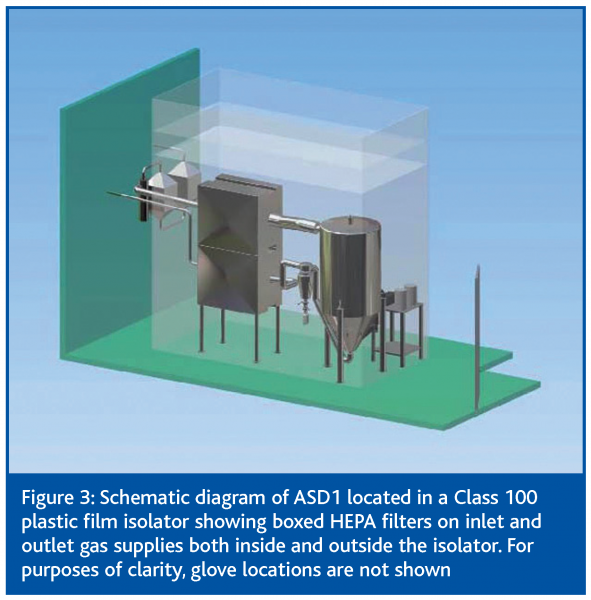

The facility is designed to be able to produce sterile batches of cGMP grade SLV. Each production run will necessarily include a proportion of time for assembly, sterilisation, production and cleaning. It is planned that up to 30,000 doses will be produced per run and that one run per week may be performed. However due to the continuous nature of the spray drying process larger scale production runs would be possible.
The facility is currently being used for the transfer, scale-up and validation of a process for the production of cGMP material for pre-clinical studies leading to phase 1 clinical trial.
Controlled release
Another key challenge is to provide lasting protection from a single shot removing the need for booster vaccinations. It is estimated that removing just one of the boosters from the UK’s childhood vaccine schedule could save the NHS more than £23m.
Most vaccinations require a booster of one form or another. The WHO recommends a minimum of 18 different injections to give protection for seven childhood diseases. In the USA the Department of Health and Human Services (Centers for Disease Control and Prevention) recommends 25 doses for 14 separate diseases. This number is only going to rise with the introduction of new vaccines against a number of different diseases.
However, it is often difficult for health visitors to ensure that all children complete the course of treatment and receive full protection. In particular, compliance with vaccination schedules in Developing Countries is widely acknowledged to be poor. Many factors contribute to this problem including misinformation about the need for boosters, fear of needles and the logistical burden on the patient in travelling to the health centre. It is therefore clear that a new approach to vaccination programmes is required.
If it was possible to provide an effective slow release of all vaccines, the stimulation of the body’s immune system would be achieved over a longer time and so full protection may be achieved from a single injection. In this way benefits would be expressed in both economic terms and in the level of protection found in the population as a whole.
CBL’s technology stabilises vaccines so that they are inert by encapsulating them in tiny glass microspheres, which dissolve in the body fluids after injection. It is thought that by introducing CBL’s proprietary nanoparticles (consisting of a form of calcium phosphate) within the microsphere, the vaccine release will be slower.
The vaccine will be released into the body over a longer time period so protection will be prolonged. The exact processes which are causing this observed effect are not clear. It is assumed that the body’s own mechanisms responsible for the constant re-modelling of bone are able to slowly break down the calcium phosphate microspheres – thus releasing vaccine over an extended period of time. As each microsphere is an individual entity isolated in the anhydrous suspending fluid and cannot therefore interact with any other, then heterogeneous mixtures of microspheres may be combined in a single shot. Thus the intriguing possibility of mixing formulations of vaccines with a variety of release kinetics may become a possibility in the future.
To this end, a research consortium has been formed to develop this technology. Funding for the research of £1.5 million has been awarded under the Micro and Nanotechnology Manufacturing Initiative for a three year project. CBL is the lead partner in the consortium; which includes MNLpharma (MNL), who will provide novel adjuvants for incorporation into the formulation and the Multi-Imaging Centre at the University of Cambridge (MIC), which will analyse the nanostructures which are formed. The aims of the project are to determine the nature of the physical and immunological processes underway during slow release of SLVs. In this way the processes may be adjusted and eventually adapted to a wide range of suitable vaccines.
Conclusion
To create and maintain stocks of vaccine is problematic at present, whether this is to support emergency response to natural disasters, bio-defence strategies for urban areas, or as part of a global response to eliminating preventable diseases; momentum to solve these issues is growing. Additionally, considerable sums of money are being directed at finding solutions both from the bio-defence initiatives and philanthropic campaigns.
As described above CBL has developed a number of technologies which offer the potential to revolutionise the storage and transportation of vaccines. The challenges in the near future will be to develop the existing applications beyond the research environment and into manufacturing.
References
- http://www.vaccinealliance.org/General_Information/ Immunization_informa/Diseases_Vaccines/vaccine_preventable_deaths.php
- http://www.vaccinealliance.org/resources/12_board _techrep.pdf
- Hepatitis B vaccine freezing in the Indonesian cold chain: evidence and solutions. Nelson CM, Wibisono H, Purwanto I et al. Bulletin of the World Health Organization, February 2004, 82 (2):99-105.
- http://www.who.int/vaccines-documents/DoxNews/updates/updat34e.pdf
About the author – Howard Smith
Howard Smith has worked in the biotechnology vaccine industry since 1991 across a variety of fields. Initially working on veterinary vaccines against intestinal parasites, he joined Axis Genetics to develop novel vaccine expression systems using plant viruses and transgenic food crops. He was head of Biopharmaceutical Analysis at NDA Analytics, where his team provided contract cGLP/cGMP analytical and stability services. In this role he gave a number of presentations at international conferences on the design and performance of cGMP analytical processes. Howard joined Cambridge Biostability in April 2004 to co-ordinate the research process. He has subsequently taken on additional responsibilities in the commercial activities of the company.









