The AstraZeneca ACMF
Posted: 22 August 2005 | | No comments yet
Compound management is an emerging discipline that represents a core component of the drug discovery process, from early phases involving high throughput screening (HTS) to late-stage lead optimisation screening cascades. This Compound management represents the integration of automation, database management, software engineering, process design and optimisation and chemistry expertise. Successful compound management groups today have […]
Compound management is an emerging discipline that represents a core component of the drug discovery process, from early phases involving high throughput screening (HTS) to late-stage lead optimisation screening cascades.
This Compound management represents the integration of automation, database management, software engineering, process design and optimisation and chemistry expertise. Successful compound management groups today have a clear understanding of the drug discovery process and a strong customer focus, with adaptable approaches to meet project needs. Key responsibilities of compound management include inventory of the corporate compound library, maximising and monitoring stability and integrity of hundreds of thousands of diverse chemical compounds and sample processing with high efficiency and accuracy.
Challenges in compound management have been steadily increasing due to larger corporate collections, greater complexity in Lead Generation processes and industry pressures to optimise time and cost efficiency. Assay technologies are evolving at a rapid pace, demanding a wider variety of plasticware, plate formats and dispense volumes. To address challenges such as sample integrity, accessibility, throughput and efficiency, the industry has moved towards large-scale automation. Many companies have established automated compound management facilities (ACMF), designing a wide variety of solutions. Taking sample storage as an example, systems range from large building-sized stores that require a specialised civil engineering project, to modular mobile storage units; storage temperatures vary from -80°C to room temperature and formats range from master copy/multiple uses to multiple copies/single use approaches. Most of these systems are focused on sample supply for high throughput screening1, 2, 3.
To maximise the integration and exploitation of compound management processes in drug discovery, AstraZeneca has built a global network of Automated Compound Management Facilities (ACMF)4, with a centralised compound store at Alderley Park, UK. Four satellite facilities are operated within Research Areas dedicated to specific therapeutic indications. Compound accessibility and sample quality were identified as key focus areas – both globally and locally – and integrated into a global network of facilities with standardised units for maintaining compound quality and customised processing capabilities to meet individual Research Area needs. These characteristics are consistent with the dynamic nature of the collection, which is expected to evolve with the inevitable sample depletion and with the changing definition of ‘lead-like’ properties for compounds to be screened.
The CNS/Pain ACMF in Wilmington, DE
The Wilmington ACMF has both local and global deliverables. It is responsible for compound supply from the liquid library collection to the local psychiatry portfolio projects and is an integral component of the global CNS/Pain HTS center, one of four HTS centers in AstraZeneca. It also provides samples to the other CNS/Pain research sites in Montreal and Södertälje, Sweden and to the remaining North American research site in Waltham, Massachusetts.
Large-scale automation is a major investment, with an expected useful life of more than ten years. Sample storage solutions when designed properly can be relevant for the long term, however, sample processing approaches require constant updates to remain compatible with changing technologies and screening strategies. Project teams rely on ACMF deliverables not only to support HTS, but also to support higher throughput in vitro ADME assays, virtual screening approaches that result in large compound sets for testing and even scaffold exploration in lead optimisation projects. High capacity subsetting is demanded in each of these areas with compound sets ranging from 5 – 30,000 compounds, for screens aimed at specific targets as well as to provide profiling data for the development of biological activity models. Both compound integrity data and compound concentration information are required to ensure accurate interpretation of screening results. Another challenge, in particular in the CNS/Pain area, is the wide use of cell-based assays – in fact, more than 70 per cent of all HTS assays run in Wilmington since 2000 have been in cell-based formats. This requires very low DMSO concentrations, imposing restrictions on the volume of compound delivered. In the next section, we will describe the components of the Wilmington ACMF system and how it has functioned in the first six months post-launch.
In designing the facility, compatibility with the global AstraZeneca compound network was essential, but a number of additional considerations have resulted in a partially customised design to best meet the project needs. First and foremost was a requirement for flexibility to meet a wide range of requests, delivering samples in assay ready formats for high efficiency in HTS, secondary screening and ADME analyses. Output formats can vary, for example, from 96 to 384 to 1536; from single poke to triple or quadruple to serial dilution, with different filling patterns within a plate. Providing samples in an assay ready format eliminates the need to perform further dilutions or reformatting before testing and greatly reduces the waste of library samples. In most cases, samples are provided on demand for all local customers, eliminating freeze/thaw cycles and the need for customer plate storage.
The ACMF Building
The Wilmington ACMF is housed in a 16,000 square-foot building specifically designed to meet the system requirements. The building has two stories; the first floor encompasses the laboratory where all the automation equipment is located and the second floor provides space for offices and building mechanical equipment. Large capacity air handling systems were installed to maintain the required environment of the main storage and processing area. A fully automated DMSO system was implemented to pump DMSO from three chained 200-liter drums in a dedicated DMSO storage room to all sampling processing systems and waste liquid is taken back to waste drums in the same room. The roof of the building is fitted with solar panels that supply up to 25,000 kilowatts-hour of power to the building per year (Figure 1). This is equivalent to planting 1000 trees or eliminating 1.6 million miles of driving.
Compound Storage and Retrieval Systems
The Primary Liquid Store
The Primary Liquid Store (PLS) was purchased from RTS Life Sciences (Irlam, UK). The function of the PLS is to house our master liquid sample collection. A detailed description of the PLS can be found elsewhere (Spencer, 2004). Briefly, the PLS consists of a main store with a capacity of 4 million tubes, a picking area that has 4 flex-picker robots that can pick and place more than 35,000 tubes a day and a conveyor that transports samples in and out of the store. Each sample is solubilised in neat DMSO and contained in a bar-coded, air -purged standard vessel (SV) from ABgene. The SVs are collected in arrays of 96 in racks with a standard microtiter plate footprint and sent to the PLS through its input buffer via a conveyer. The conveyance delivers the rack to the flex-pickers where SVs are picked and placed into trays that can hold 2000 SVs per tray. The filled tray is then sent to the main store and empty racks are sent out of the output buffer of the conveyer. When samples are needed, a picking list of SVs is sent to the PLS, the PLS picks the requested SVs to a rack in a specified pattern and sends the rack out of the system for processing. Processed SVs are sent back to the PLS for storage.
The Working Plate Store
The working plate store (WPS) was purchased from TekCel (Hopkinton, MA, USA). The main purpose of the WPS is to store the set of HTS mother plates. The WPS consists of five PlateStores and two PlateServers. Each PlateStoreTM can store more than 900 standard plates. Each plate is stored with an individual mechanic lid called SealtiteTM, which provides an air-tight seal isolating the samples in each well from ambient environment and humidity. When a plate is to be stored, it is placed in the hand-off arm of a PlateServer. The plate server takes in the plates, automatically places a clear SealTite lid onto the plate and then sends it to the store. When a plate is requested, the PlateServer will pull the requested plate from the store, remove the lid and hand out the plate. When the plate is returned to storage, the exact same lid will be placed on the plate. Temperature, humidity and oxygen level can be controlled inside the Working Plate Store.
Sample processing systems
The Wilmington ACMF sample processing systems are based on a system architecture developed in-house called the AUS (Automated Universal System) that incorporates function-specific instrumentation, referred to as ‘functional components’, which are purchased from commercial vendors. A functional component can be very simple (such as a plate sealer), or very complicated (such as the Primary Liquid Store). Each functional component performs a well-defined task (such as tube/plate storage, labware transporting, liquid transfer, barcode printing, etc.) independent of the other components. AUS integrates the functional components in such a way that they are truly plug-and-play. The Wilmington AUS system has more than a dozen components incorporating over 30 different instruments. When an order is sent to AUS it will engage the required components and provide synchronised instrumentation control to most effectively produce the required final products. Different orders can utilise different components within the system. Based on the customer requirements, several AUS systems were implemented within the Wilmington ACMF and are described below.
The Reformator
The purpose of the Reformator is to process those orders that involve the SV tubes in the primary liquid store. The Reformator is mainly used to transfer samples from the SV tubes to plate formats. It integrates the PLS, which is the storage unit for SVs; a Vprep liquid handler from Velocity11; the WPS containing HTS mother plates and many other components such as barcode printers, plate sealers, stackers and bulk reagent dispensers. The Reformator is housed in an environmentally controlled enclosure where temperature is set at 18-20 degrees and humidity at 10 per cent. The Reformator produces HTS mother plates, cherry picked retest plates, single poke non-HTS assay plates and source plates for dose-response plates.
The Replicator
The main tasks for the Replicator are to make assay ready plates from mother plates for HTS, as well as produce IC50 assay plates. It uses a 384-well format for its source, mostly HTS mother plates, to produce assay plates with 96, 384 and 1536 formats. Major functional components in the Replicator include the WPS, the Echo550 liquid handler from LabCyte (Sunnyvale, CA, USA) and the Hummingbird liquid handler (Genomic Solutions, Ann Arbor, MI USA). Both the Hummingbird and Echo550 can be used to replicate assay plates with typical output volumes of 50 to 200 nl. The Replicator can produce more than 400 assay plates a day.
Since the Echo550 is capable of transferring volumes as low as 5 nL of liquid accurately, we have also created an alternative way to produce IC50 plates with higher accuracy (described in Cesarek & Nie, 2005). The Replicator is responsible for that task.
The Distributor
The purpose of the distributor is to make plates for heavily customised sample requests. Main components in the distributor are liquid handlers with tips that operate independently. Larger volume (10 ul or higher) requests are carried out using TECAN Genesis and low volume (0.1- 10 ul) requests by the Leap HTC PAL. While the throughput is low, the distributor can produce plates with unusual formats, such as randomised replicates of samples.
Operations and sample flow
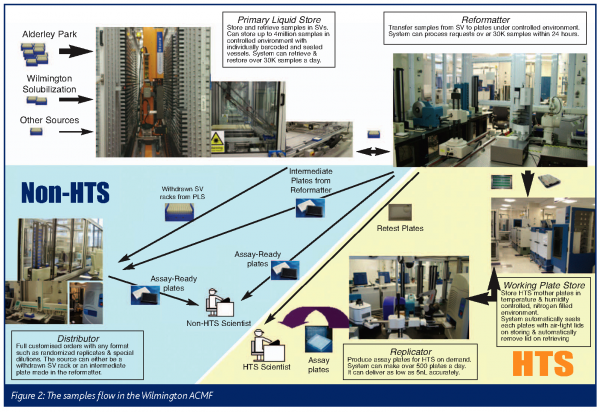
The Wilmington ACMF operations support all drug discovery activities in the research area. Figure 2 shows the samples flow for sample supply to HTS and non-HTS customers.
Liquid samples, contained in SVs, are loaded into the PLS ready for use. The PLS maintains a temperature of 18 degrees to prevent the samples from freezing. Humidity in the PLS is maintained at ten per cent. Samples in the PLS correspond to legacy compounds synthesised in-house, as well as compounds acquired from external sources and purified natural products. It is expected that the liquid library will be replaced every five years, or when it is depleted.
When samples are needed, scientists can make requests from their office PC (or any PC in the company), specifying their requirements according to sample IDs, volumes, output format, etc. Requests are sent to a global compound order system (COSMOS) where availability is checked and reserved. The request is then retrieved by a local application called WAUSPS where the appropriate AUS systems are selected and jobs are generated for the selected AUS to process the request. ACMF operators monitor jobs received by each AUS system and find all new jobs created for that system listed in the order of priority. The operator can than select and start a job.
In the case of HTS, samples are grouped into focused subsets based on structural features associated with biological properties of particular target classes (e.g. GPCRs) or designed for maximum structural diversity. According to this subsetting scheme, mother plates are created in the Reformator and stored in the WPS. Mother plates have a life expectancy of one year. HTS scientists can request assay plates by specifying either individual mother plates, subsets, or the entire library. Assay plates will be produced in the Replicator, where mother plates are retrieved from the WPS for replication. Assay plates are made on demand and delivered to the scientist according to the specified timelines. When biologically active compounds are identified, the list of the SVs containing the active compounds is sent to the PLS to pick and the cherry picked plates are produced in the Reformator on demand.
When samples are requested for serial dilutions, a source plate is prepared in the Reformator and then carried to the Replicator. Assay ready plates are made with the Echo550 in the Replicator for both HTS and non-HTS screening. Most other requests for non-HTS assays are filled in the reformator, although heavily customised requests involve the Distributor. Highly customised requests include plate formats with randomised duplications and orders requiring positioning of samples in specified wells of an output plate map. If completing a request requires functionality that is not currently available, the system administrator will add the required functional components to the system, allowing the job to be created and carried out in the system.
A quick reality check
The Wilmington ACMF has been in operation since its commissioning at the end of 3Q 2004. While there have been some key learning experiences to begin with – and additional upgrades remain to be implemented – the facility has generally performed satisfactorily. So far it has successfully filled more than 200 requests originating from all North American & CNS/Pain Research Area sites within required delivery timelines. The largest single request so far has been for 19,000 samples, which was completed within a 12 hour period. ACMF has successfully supported three HTS campaigns simultaneously, with assay ready plates produced on demand. The large cherry picking capacity of the system now allows project teams to access HTS active samples for re-testing within 24 hours, as compared to the several week delivery-time required before ACMF implementation.
Looking forward
ACMF is designed to address the major compound management issues facing the industry today such as sample integrity; delivery accuracy and efficiency; storage capacity and process capability. It is focused on meeting the current and future compound requirements of drug discovery activities. The four AstraZeneca ACMFs are coordinating a large scale effort to monitor the quality of the liquid samples by periodic analysis using LC/MS and in Wilmington we are in the process of implementing methods to measure the concentrations of all samples delivered to project teams. By providing effective service and timely delivery, together with accurate quality assessment data for the compound samples, ACMF promises to significantly accelerate project progression across all discovery phases.



References
- Harrison, W. Changes in scale in automated pharmaceutical research. Drug Discovery Today 3, 7, 343-349 (1998),
- Wood, T, and Keighley, W. Automated sample supply for high throughput screening. European Pharmaceutical Review 9 1, 68-73 (2004).
- Archer, R. History, evolution, and trend in compound management for high throughput screening. Assay and Drug Development Technology 2, 6, 675- 681 (2004)
- Spencer, P. The challenges of managing a compound collection. European Pharmaceutical Review 9 2, 51-57 (2004).









