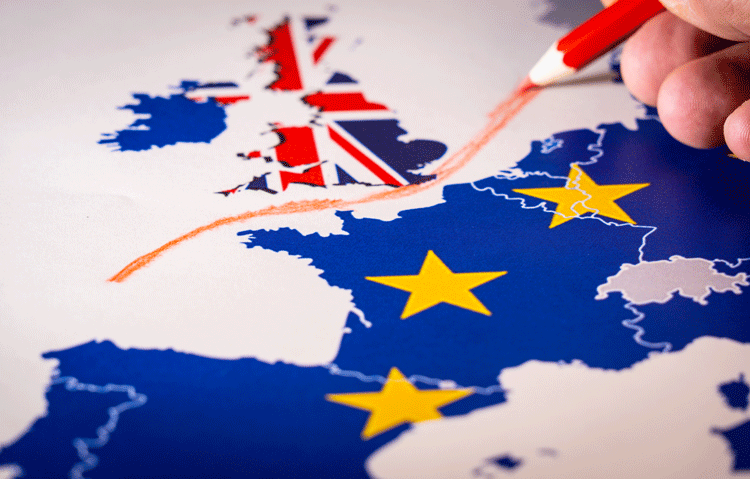
Is the pharma sector ready for a ‘no-deal’ Brexit?
With the UK bracing itself for a 'no-deal' Brexit or some other outcome that has not yet been determined, pharma experts discussed the potential effect of Brexit on the supply chain and how to best prepare for it against the upcoming deadline.