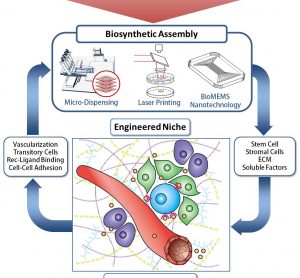
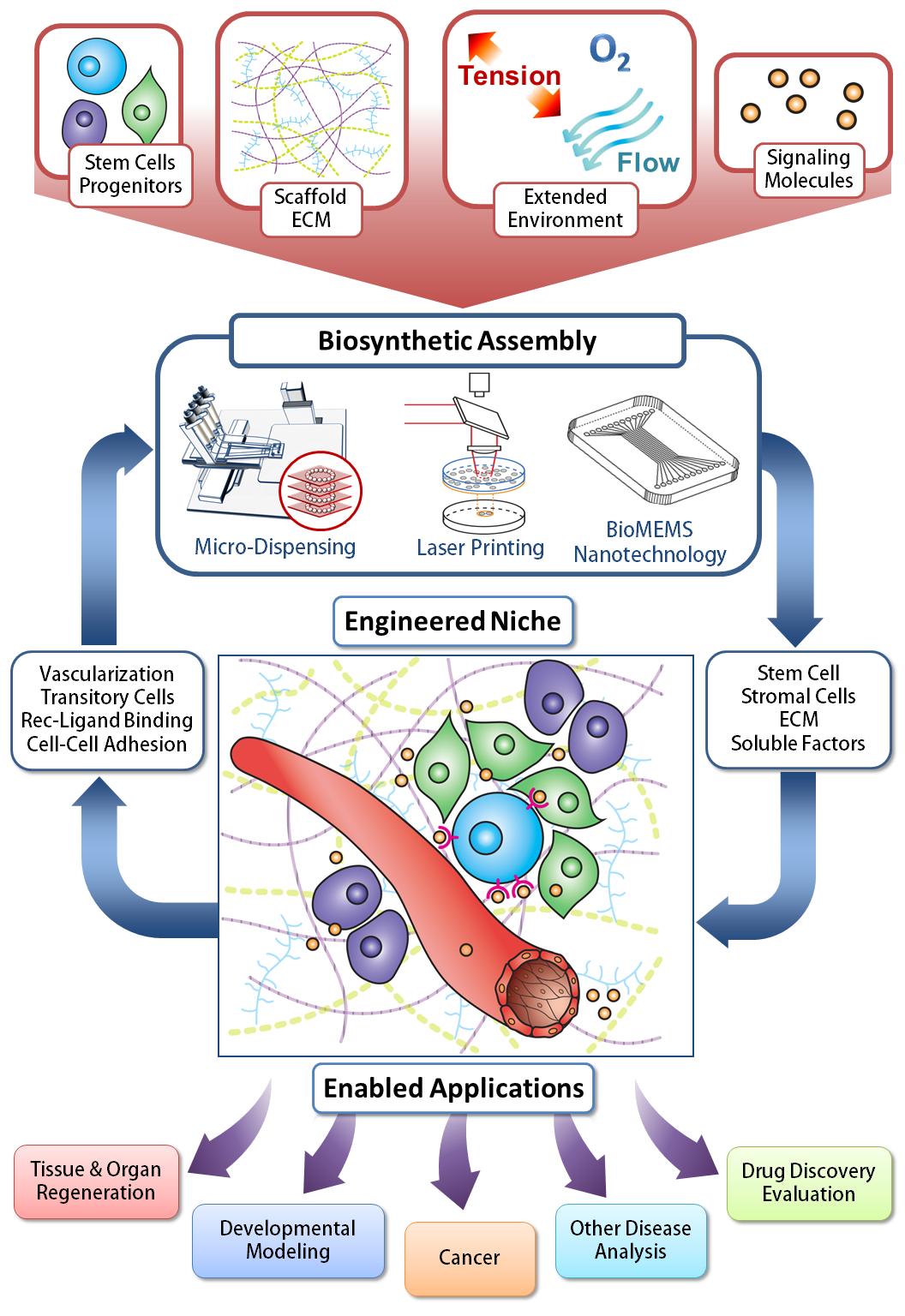
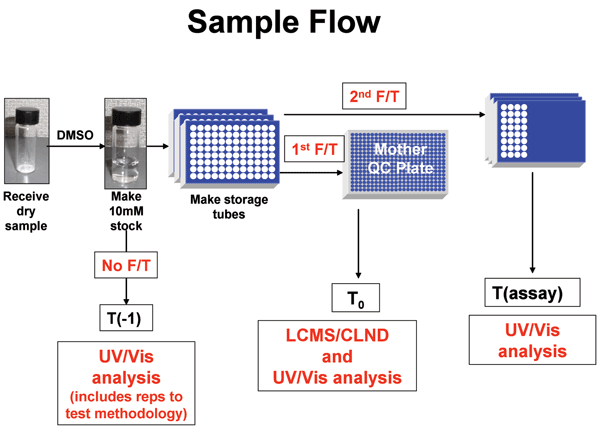
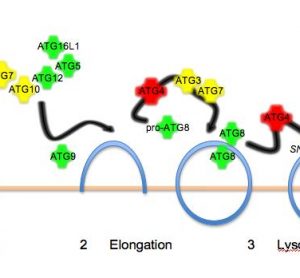
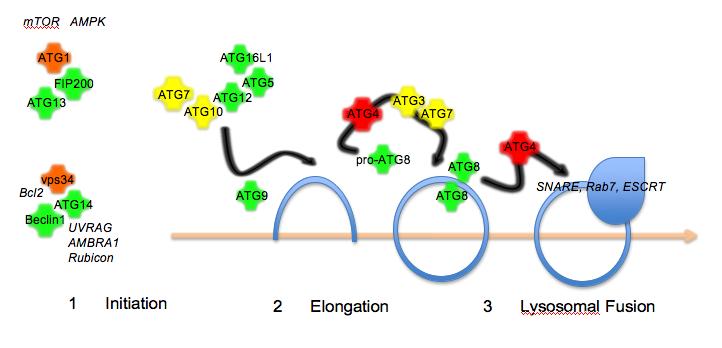
In search of the Holy Grail: Engineering the stem cell niche
19 April 2011 | By Janet L. Paluh, Associate Professor Nanobioscience, College of Nanoscale Science and Engineering, University at Albany SUNY and Guohao Dai and Douglas B. Chrisey, Biomedical Engineering, Rensselaer Polytechnic Institute
There is no other biomedical frontier that offers the stunning potential of human pluripotent stem cells and their progenitors in therapeutic applications to ease human suffering or in their ability to provide insights into development and diseases. Cell plasticity for reprogramming has revealed new opportunities in cell-based therapies and informed…