Report links greater generic competition and lower generic drug prices
Posted: 16 December 2019 | European Pharmaceutical Review | No comments yet
A new report from the FDA has shown that greater competition among generic drug makers is associated with lower generic drug prices.

Greater competition among generic drug makers is associated with lower generic drug prices, according to a new analysis using two different sources for wholesale prices, the US Food and Drug Administration (FDA) has announced.
In a new report, the regulatory body shows that generic drug prices after initial generic entry decline with the additional competition using both the average manufacturer prices (AMP) reported to the Centers for Medicare and Medicaid Services (CMS) and invoice-based wholesale prices reflecting pharmacy acquisitions from IQVIA’s National Sales Perspective database (NSP).
Estimates using AMP show price declines associated with the additional generic competition are steeper than those based on invoices for pharmacy acquisitions, though most of the difference comes from wholesaler markups, the agency said.
The FDA’s analysis of prices and competition for all drug products that had initial generic entry between 2015 and 2017 can be found below, showing median generic-to-brand price ratios and their ranges by the number of generic producers.
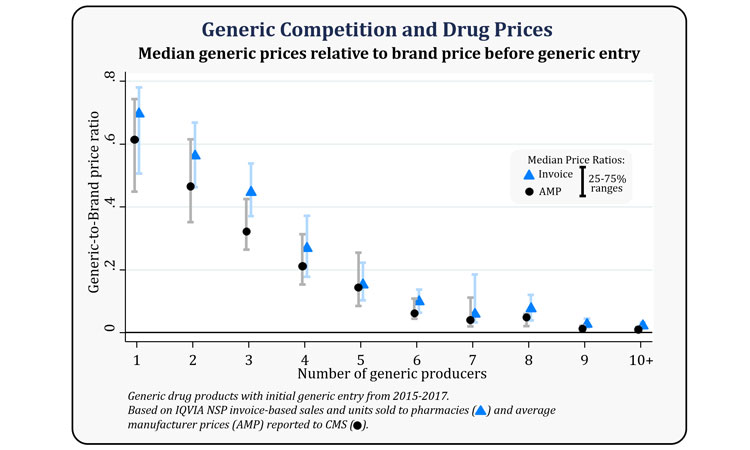

Credit: FDA.
The report has found that for products with a single generic producer, the generic AMP is 39 percent lower than the brand AMP before generic competition, compared to a 31 percent reduction using invoice prices. With two competitors, AMP data show that generic prices are 54 percent lower than the brand drug price before generic competition, compared to 44 percent when calculated using invoice-based drug prices.
With four competitors, AMP data show that the generic prices are 79 percent less than the brand drug price before generic entry, compared to 73 percent when calculated using invoice-based drug prices. With six or more competitors, generic prices using both AMP and invoice prices show price reductions of more than 95 percent compared to brand prices.
Combining all competition groups, the FDA has said it has found that the median price of generics relative to brands using AMP is 40 percent for the drugs in its sample, while the median price ratio using invoice prices is 49 percent.
The FDA has said this analysis builds on its earlier work comparing generic and brand drug prices and follows related studies.
Related topics
Big Pharma, Data Analysis, Drug Markets, Generics, Industry Insight, Regulation & Legislation, Research & Development (R&D)