Influenza antiviral Avigan® (favipiravir) to enter Phase III trials in COVID-19 patients
Posted: 3 April 2020 | Hannah Balfour (European Pharmaceutical Review) | 2 comments
The developers of Avigan have increased production and announced a Phase III clinical trial testing its efficacy against COVID-19.
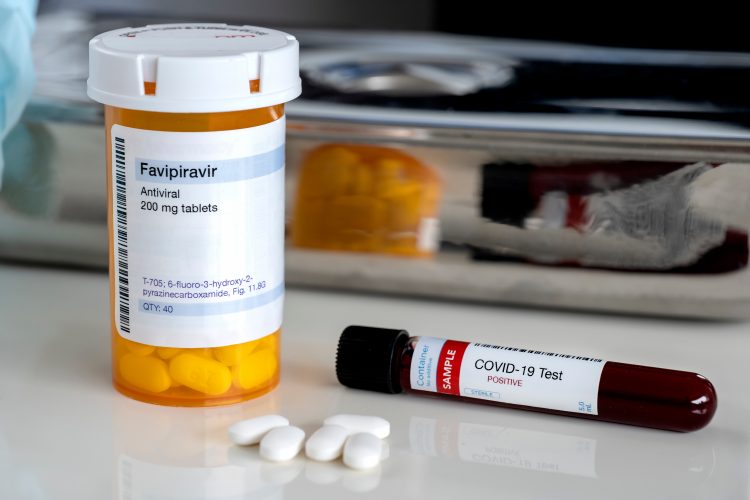

FUJIFILM Toyama Chemical Co. Ltd. have announced a Phase III trial to evaluate the safety and efficacy of its influenza antiviral drug, Avigan® Tablet (favipiravir) for patients of COVID-19.
Avigan is approved for manufacture and sale in Japan as an influenza antiviral. It selectively inhibits the RNA polymerase of the influenza virus, an enzyme required for viral replication once human host cells are infected. COVID-19 also uses this enzyme to replicate and is classified into the same type of single-stranded RNA virus as influenza; hence, it is believed that Avigan may be effective in treating COVID-19.
Avigan is only used when there is an outbreak of novel or re-emerging influenza virus infections in which other influenza antiviral drugs are either not effective or insufficiently effective. Its production and distribution is at the discretion of Japan’s Health, Labor and Welfare Ministry, so has never been distributed in the market and is not available at hospitals and pharmacies in Japan or overseas.
Fujifilm states it has already begun increased production of Avigan and plans to accelerate the production through co-operation with domestic and overseas partners. The enterprise intends to supply both the Japanese government and other countries to help tackle the spread of the COVID-19 pandemic.
Related topics
Clinical Trials, Distribution & Logistics, Drug Manufacturing, Drug Markets, Industry Insight, Research & Development (R&D), Therapeutics
Related organisations
Fujifilm, FUJIFILM Toyama Chemical Co. Ltd., Japan's Health Labor and Welfare Ministry







what are the outcomes of clinical trials so far?
in my mind, succesfull antiviral treatment based on use viral or bacterial antagonism themselves. When using a competative inhibitor, the efect will be less specific and unstable