Dolomite’s microfluidics technology ideal for B cell encapsulation
25 March 2015 | By kdm communications
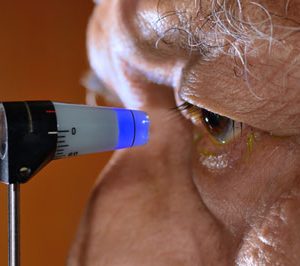
A Dolomite droplet generation system is helping researchers at Weill Cornell Medical College in New York, USA, to encapsulate human and mouse B cells for the cloning of antibody genes...