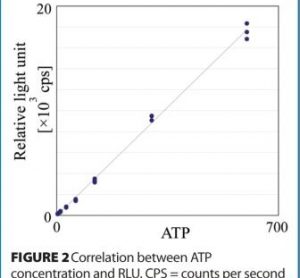
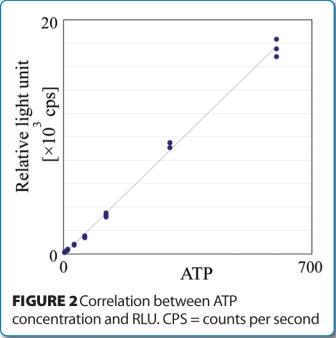
The regulatory acceptance of rapid microbiological methods
In the last issue of European Pharmaceutical Review (vol 22, issue 2), Jeanne Moldenhauer suggested the pharmaceutical industry has moved at a “snail’s pace” in terms of implementing rapid microbiological methods (RMM).