Controlled nucleation in freeze-drying
22 October 2012 | By Henning Gieseler, Associate Professor at the Division of Pharmaceutics, University of Erlangen & CEO, GILYOS GmbH and Peter Stärtzel, Pharmaceutical Scientist, GILYOS GmbH
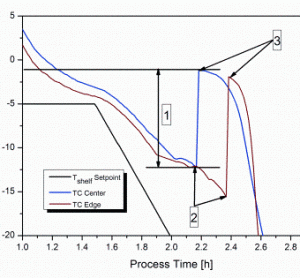
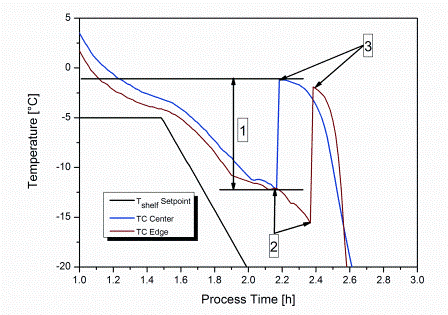
The stochastic nature of nucleation during the freezing step of the freeze-drying process has been regarded as a demerit in a process which is considered under rigorous control. The freezing performance of a product can impact its subsequent drying behaviour and the final product quality attributes. Hence, the idea to…