The role of liquid handling technologies in successfully executing screening campaigns
Posted: 3 December 2008 | Dr Sheraz Gul, Vice President and Head of Biology, European ScreeningPort GmbH | No comments yet
High Throughput Screening (HTS) has for many years now been playing a central role in drug discovery efforts to aid the identification of small molecule chemical entities that are capable of modifying the activity of disease relevant targets1. In order to make HTS a viable option to provide appropriate starting points for drug discovery efforts, large libraries of compounds are required that contain diverse chemical space.
High Throughput Screening (HTS) has for many years now been playing a central role in drug discovery efforts to aid the identification of small molecule chemical entities that are capable of modifying the activity of disease relevant targets1. In order to make HTS a viable option to provide appropriate starting points for drug discovery efforts, large libraries of compounds are required that contain diverse chemical space.
High Throughput Screening (HTS) has for many years now been playing a central role in drug discovery efforts to aid the identification of small molecule chemical entities that are capable of modifying the activity of disease relevant targets1. In order to make HTS a viable option to provide appropriate starting points for drug discovery efforts, large libraries of compounds are required that contain diverse chemical space.
These libraries are typically composed of 0.5 to 3.0 million distinct compounds in solution (usually DMSO) of which 10,000 to 100,000 are available in solid form. Subsequent to the execution of an HTS at an appropriate single concentration of compound, thousands of these are typically identified and are classified as actives. Some of these initial actives may be false positives, therefore their activities are usually confirmed in independent experiments carried out in duplicate followed by dose-response experiments to determine their potencies. The final set of confirmed actives is termed validated hits. Appropriate selectivity and liability assays enables annotation of these compounds and the most promising ones can be considered for structure-activity-relationship (SAR) studies. The SAR process is an iterative process in which new compounds are synthesised, their activities determined in appropriate assays, followed by further synthesis and compound profiling. Upon achieving the desired properties in the compound, potential starting points (lead like molecules) are obtained which would undergo a series of in-vivo validation studies. Subsequent lead optimisation could result in a pre-clinical candidate molecule which would enter a period of efficacy, safety and absorption, distribution, metabolism, excretion and toxicity (ADME-Tox)2 testing in animals and ultimately enter the various human clinical studies. The time period for a drug discovery program from its conception to initiation of human clinical trials is typically in excess of five years and consumes tens of millions of dollars. This article will overview how liquid handling technologies have aided the successful development of screening compatible assays.
Biochemical assays have traditionally been considered to be sufficiently robust for HTS purposes3. The identified actives from biochemical assays would subsequently be screened in more physiologically relevant low throughput cell based assays. It is now becoming more common to use the cell based assays directly in HTS campaigns. This has however, required appropriate miniaturisation of the assays to overcome the issue of reagent supply. In the case of generic reagents that are available in large quantities such as buffer components, the increase in their consumption would not be an issue. However, in the case of biological reagents, which include proteins, cell lines, synthetic substrates and detection reagents, significant increases in the requirements for these reagents could make a screening campaign inhibitory in financial terms and also with respect to the inability to generate such large amounts of biological reagents. Reducing assay volumes results in corresponding savings in reagent consumption. Moving to low volume assays introduces challenges such as increases in the surface area-to-volume ratios which would result in an increase in the chances of evaporation. The behaviour of assays cannot, therefore, always be easily predicted.
The role of automated liquid handling in screening campaigns
There have been major advances with respect to liquid handling technologies such that it is now possible to purchase appropriate equipment with a relatively small budget (< £15,000) that are capable of operating with the appropriate level of integrity, reliability and reproducibility4-7. The control software is of particular importance as many instruments now operate using a Windows based interface that most users can easily become familiar with8. The size of the equipment has also decreased considerably such that there are many available that have a footprint of a small printer. It needs to be borne in mind that there needs to be a pragmatic approach to the role of automation when applied to screening campaigns. The choice of liquid handling system should be made after careful assessment as to the overall benefit it will provide and should be made on a case by case basis. Much of this will be dictated by the number of compounds that need to be assayed, micro titre plate density, reagent supply and the timescale for completion of the screening campaign. For illustrative purposes, an HTS executed in 384 well format (micro titre plates with 24 columns and 16 rows, with one column each for high and low controls for plate quality control9 and Z’ determination10) with a volume of 10µl per well would allow 352 unique compounds to be assayed per micro titre plate. To screen a library of one million compounds in singlicate would consume 2840 of such micro titre plates. If a throughput of approximately 150 micro titre plates per day could be achieved, the campaign could be completed in approximately 19 working days. The total reagent consumption would be approximately 11 litres. If the same HTS was executed in the higher 1536 well format (micro titre plates with 32 rows and 48 columns, with two columns each for the high and low controls) the statistics are considerably different: for the same one million compound screen, 710 micro titre plates would be required consuming approximately five litres of reagent. With a throughput of 150 micro titre plates per day the screen could be completed in less than five working days. It is therefore apparent that miniaturising an assay to the 1536 well format provides major advantages over the lower 384 well density assay format with respect to reagent consumption, overall cost and time. Miniaturisation to this extent would also ensure that the running of a HTS is not the bottleneck for the overall early drug discovery process. Indeed, it may be shifted to other phases such as compound dispensation for the HTS itself, data analysis and cherry picking of compounds for further confirmation screening activities. A summary of the above can be found in Table 1.
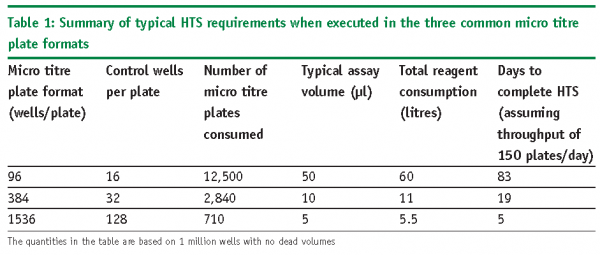

The requirements in Table 1 are the minima and do not include the dead volumes required for priming of the equipment, failed runs that need repeating, follow up single concentration confirmation screening and dose-response experiments to determine their potencies. Miniaturising assays therefore provides multi-faceted benefits. This has been made possible with the design, development and manufacturing of liquid handling equipment capable of delivering assays in 1536 well format. The two main types of dispensing technologies are now described together with their advantages and disadvantages.
Contact dispensers
These types of dispensers involve ejecting the liquid whilst the tip is touching the substrate it dispenses into. Contact between the destination surface and the ejected liquid from the dispensing tips is necessary as the liquid is not released with sufficient momentum. This requirement for contact results in limitations of its potential applications. In particular, this will dictate the sequence in which reagents can be dispensed and has the potential of cross-contamination. Overcoming this problem can be accomplished by either the introduction of wash steps or changing the tips, both of which can be time consuming and has cost implications. Most contact dispensers utilise a “head” that typically contains disposable tips that are pre-aligned in either a 96 or 384 well format. These tips aspirate the liquid after which the head is relocated to the destination where the contact based ejection of liquid occurs. Other types of contact dispensers utilise syringes that are connected to pumps. The dispense syringes are connected to a stepper motor that drives the syringe plunger and pushes the liquid to its destination.
Non-contact dispensers
One of the early technologies that is still currently available, that is based on the non-contact principle, is the Genomic Solutions synQUAD. It uses the opening and closing of a solenoid valve in conjunction with the movement of a high resolution syringe pump to dispense droplets from a ceramic tip. Some systems have a flow-through design where the liquid from the reservoir enters the dispenser and travels the entire length of any tubing in the dispenser, with the fluid being ejected from the other end11. The synQUAD, although capable of dispensing in the low microlitre volume range requires significant investment in time in setting it up appropriately. Other types of dispenser exploit inkjet technologies used in the printing industry (piezoelectric dispensing)11,12. The Thermo micro-multidrop Combi nL has been recently launched and has superior qualities for liquid handling. It is capable of dispensing volumes from 50nl to 50µl per well in the three common micro titre plate types listed in Table 1 on page 52. It can dispense in either rows, columns or individual wells. Its simple peristaltic mechanism uses a cassette encasing silicone tubing with eight channels that sweeps left and right across micro titre plates to dispense reagents. The dispenser is capable of dispensing most biological reagents including soluble proteins, membrane preparations and a variety of cell types (bacterial, mammalian and insect origin). It is also compatible with most of the commonly used solvents such as DMSO at concentrations used in assays, buffers, viscous solutions, as well as non-biological reagents such as synthetic substrates, peptides and beads for SPA and ALPHAlisa assays. For those reagents that are susceptible to binding to the silicone tubing inclusion of an appropriate detergent in the assay buffer usually obviates this problem. As the limits of current mechanical devices is rapidly reaching their limits in terms of having the potential to dispense ever smaller volumes, advances are being made in micro-fabrication technologies and these are being exploited in the drug discovery world13-15.
Conclusions
Screening activities in drug discovery have undergone a revolution during the last decade. This has been, in part, possible due to the development of robust technologies for compound addition as well as devices capable of dispensing from bulk reagent reservoirs. Advances in various areas in automation, assay design and cell culture techniques have together enabled the possibility for scientists to choose which assay is the most appropriate for screening. The 1536 micro titre plate format has now become a standard for HTS efforts. This has been facilitated by advances in detector technologies, in particular with the advent of image based detectors that use supercooled CCD cameras capable of imaging entire micro titre plates e.g. Perkin Elmer Viewlux rather than single well readers. The Viewlux is compatible with most of the common assay technologies (luminescence, fluorescence intensity, fluorescence polarisation, fluorescence resonance energy transfer and time-resolved fluorescence). Although liquid handling equipment was developed as long as a decade ago capable of sub micro-litre dispensing in the most common micro titre plate densities (up to 1536 well) they had reliability issues and were not compatible with all biological reagent types and bead based assay technologies. The industry has however, come a long way since then. The Thermo Scientific Multidrop Combi nL is one of the latest bulk reagent dispensers that offers excellent reliability, precision and accuracy and meets low volume reagent dispensing requirements from 50nl to 50μl into 96, 384 and 1536 well micro titre plates. It has a small footprint and can be integrated with a range of robotic platforms. Acoustic dispensers have also been manufactured but have thus far been shown to be only suitable for dispensing small volumes and are now routinely used for dispensing compounds. This method overcomes issues that can be a common occurrence with both contact and non-contact dispensers as their components do have the potential to failures such as blockages of feed lines. Micro-fluidics are also being exploited in drug discovery which have the potential to overcome the limitations of the low volume liquid handling systems described herein.
References
- Liu, B. Li, S. and Hu, J. Technological advances in high-throughput screening. Am. J. Pharmacogenomics. 2004, 4:263-276.
- Welling P. G., Lasagna, L and Banakar, U. V. (eds.). The drug development process: Increasing efficiency and cost effectiveness. New York Marcel Dekker, Inc., 1996.
- Wölcke, J. and Ullmann, D. Miniaturized HTS technologies–uHTS. Drug Discovery Today. 2001, 6:637-646.
- Sundberg, S. A. High-throughput and ultra-high-throughput screening: solution- and cell-based approaches. Current Opinion in Biotechnology. 2000, 11:47-53.
- Albert, K. J., Bradshaw, J. T., Knaide, T. R. and Rogers, A. L. Verifying liquid-handler performance for complex or nonaqueous reagents: a new approach. Clin. Lab. Med. 2007, 27:123-138.
- Rhode, H., Schulze, M., Renard, S., Zimmermann, P., Moore, T., Cumme, G. A. and Horn, A. An Improved Method for Checking HTS/uHTS Liquid-Handling Systems. J. Biomol. Screen. 2004, 9:726-733.
- Rose, D. Microdispensing technologies in drug discovery. Drug Discovery Today. 1999, 4:411-419.
- Gu, H.,Unger, S. and Deng, Y. Automated Tecan Programming for Bioanalytical Sample Preparation with EZTecan, ASSAY and Drug Development Technologies. 2006, 4:721-733.
- Quintero, C., Rosenstein, C., Hughes, B., Middleton, R. and Kariv, K. Quality Control Procedures for Dose-Response Curve Generation Using Nanoliter Dispense Technologies. J. Biomol. Screen. 2007, 12:891-899.
- Zhang, J. H., Chung, T. D. and Oldenburg, K. R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999, 4:67-73.
- Niles, W. D. and Coassin, P. J. Piezo- and Solenoid Valve-Based Liquid Dispensing for Miniaturized Assays. ASSAY and Drug Development Technologies. 2005, 3:189-202.
- Lemmo, A. V., Rose, D. J. and Tisone, T. C. Inkjet dispensing technology: applications in drug discovery. Current Opinion in Biotechnology. 1998, 9:615-617.
- Dittrich, P.S. and Manz, A. Lab-on-a-chip: microfluidics in drug discovery. Nature Reviews Drug Discovery. 2006, 5:210-218.
- Wen, Y. and Yang, S-T. The future of microfluidic assays in drug development. Expert Opinion Drug. Discovery. 2008, 3:1237-1253.
- Kang, L., Chung, B. G., Langer, R. and Khademhosseini, A. Microfluidics for drug discovery and development: From target selection to product lifecycle management. Drug Discovery Today. 2008, 13:1-13.









