The impact of supply chain risks and LAL reliance
Posted: 26 June 2020 | Brendan Tindall (bioMerieux), Kevin Williams (bioMerieux) | No comments yet
For over 30 years, pharmaceutical and medical device product release has relied solely on endotoxin assays using Limulus Amebocyte Lysate (LAL). This article addresses the potential risks associated with relying on a single raw material in a fragile supply chain and explores alternative testing options.
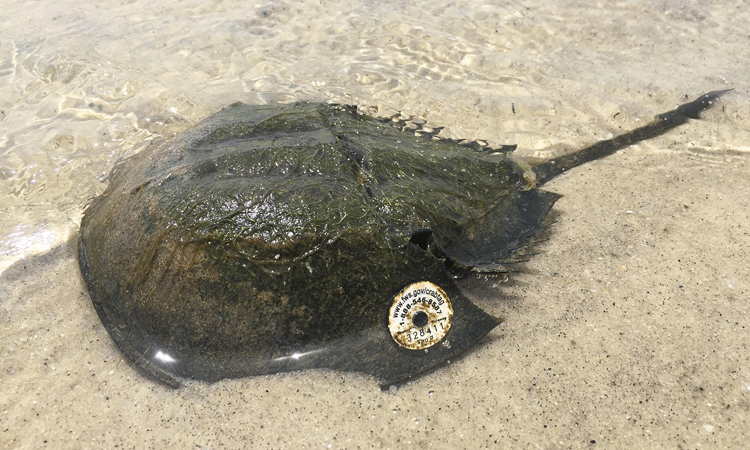

THE RELIANCE on a vulnerable or endangered animal to produce a unique reagent for endotoxin testing of drugs, vaccines and medical devices should be examined as a wake-up call for investigating and finding alternate ways to counter our dependence on this fragile segment of the overall supply chain. For over 30 years, pharmaceutical and medical device product release has relied solely on endotoxin assays using Limulus Amebocyte Lysate (LAL), as a replacement for the rabbit pyrogen test, to ensure that those products are free of harmful endotoxin. LAL is derived from only one source: the blue blood of the horseshoe crab. No other natural source exists, making this one of the rare and unique instances of a precious test ingredient being totally dependent on the continued existence and abundant supply of a single animal.
LAL deserves much credit historically for increasing medical product safety and giving increased statistical power to the detection of endotoxin contaminants in many manufacturing processes. The laborious nature of the rabbit pyrogen test used prior to LAL availability could only support a limited number of tests for a given production procedure. The advent of LAL allowed for process checks at many more test points including raw materials, waters, components, bulk materials and finished products.
There are several important and material risks associated with the availability of LAL that should obligate the development of mitigation plans to create alternative testing capabilities and avoid reliance on this single raw material in a fragile supply chain.
A partial list of potential risks associated with reliance on LAL include the following:
- horseshoe crab survival pressures
- geographic and political risks
- pandemics and other global public health crises
- recombinant factor C test availability.
Horseshoe crab survival pressures
While it is difficult to accurately track the demise of Limulus (American horseshoe crab) numbers in the US and Tachypleus (Asian horseshoe crab) numbers in Asia, there is general agreement on “diminishing populations.”1,2 It is true that some areas can be used to suggest a more steady survival in the US, but overall levels are not what they were a decade ago. In the US, Limulus is listed by IUCN as a “vulnerable” animal species and in Asia Tachypleus has been reclassified to “endangered”.3,4 The differing opinions on the numbers of remaining animals, along with the lack of numerical thresholds that would indicate alarm, gives one pause in accepting an absolute assurance of guaranteed supply.
Geographic and political risks
The current COVID-19 pandemic provides an unfortunate example of the fragility of our supply chains for vital medical products and gives us the opportunity to review our reliance on LAL from a broader risk perspective that encompasses supply and demand shocks. However, public health dangers such as epidemics and pandemics are not the only global risks that can negatively impact the availability of a precious raw material such as LAL.

In response to the COVID-19 pandemic, the United States Pharmacopeia (USP) recently published via their website the policy paper, USP Global Public Policy Position: Key Elements to Building a More Resilient Supply Chain. The paper expresses that drug manufacturers should “increase geographic diversity for ingredients and manufacturing – policymakers should incentivise geographic diversity among the sources of medicine ingredients and drug manufacturing to reduce the risk of shortages from acute disruptions that occur in one geographical location (eg, earthquake, hurricane, political disruption) or that move from one part of the world to others (eg, pandemic).”5
A more extensive list of potential exogenous geographic and/or political shocks can be conceived including, but not limited to:
(i) a significant oil spill on the Atlantic seaboard6 affecting the horseshoe crab’s environment and ultimate survival
(ii) climate-associated events such as hurricanes6 and the effects of global warming
(iii) growth of medical markets in Asia and other emerging regions where the need for more medical products and increased endotoxin testing will put additional pressures on the American horseshoe crab population9
(iv) political scenarios that promote nationalistic preference in supply designation, such as keeping LAL-derived endotoxin assays exclusively or preferentially for the US market
(v) supply halts due to quality disruptions has occurred for specific LAL manufacturers.
Pandemics and other global public health crises
Unfortunately, we have recently witnessed the effects that a global pandemic can have on medical device supplies, including an impact on the transport and availability of raw materials, import/export difficulties of raw and finished goods and even nationalistic “hoarding” – all leading to uneven distribution of vital medical supplies. These are general issues that can impact endotoxin assays as much as any other medical material but are more difficult to manage with a dependence on assays that rely on a single geographically sourced raw material like LAL.
However, there are additional risks that a global public health crisis can pose to a reliance exclusively on LAL-dependent endotoxin tests. A pandemic (eg, COVID-19), a serious epidemic (such as Ebola, Zika or MERS-CoV, for example) or a regional health crisis (eg, earthquake- and typhoon-related illnesses such as cholera and typhoid) would require a general increase in endotoxin testing.
In the case of a pandemic or epidemic with a novel pathogen, heightened endotoxin testing would be needed for new drug candidates as well as innovative vaccine candidates. In the case of an increasing disease burden following a natural disaster, additional endotoxin testing would be required for the extra medications, vaccines and devices necessary to address medical needs. It must be remembered that endotoxin testing occurs at multiple process points in the manufacture of many medical supplies, as shown in Figure 1.
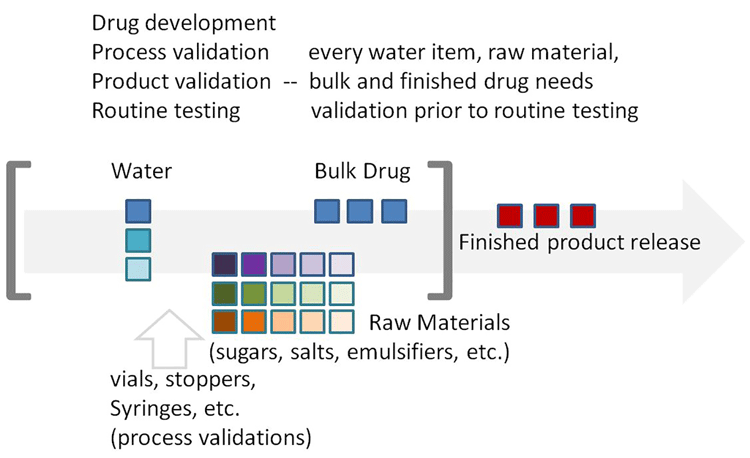

Figure 1: Each drug lot produced requires a host of endotoxin test support prior to finished product release
LAL replacement endotoxin assay availability
An existing endotoxin test that is already being used in lieu of LAL-containing assays is the recombinant factor C (rFC) test. The cloning and insertion of the gene for the natural endotoxin biosensor (factor C) from the horseshoe crab genome into single cells for mass cell culture production has enabled the production of several currently available commercial grade tests. The technology producing rFC is the same that has been used by drug manufacturers since 1982 to produce recombinant drugs including insulin and is now used to produce a myriad of drugs including monoclonal antibodies, cytokines, replacement enzymes, etc. The rFC test is analogous to LAL in that it provides the same answers (ie, EU/mL, EU/mg, etc.) and requires similar materials (instrumentation and reagent solutions) and test effort (user test performance time and manipulations) as endotoxin testing using LAL.
Several key issues have limited the uptake of rFC testing, including:
(i) Competitive challenges – while some LAL manufacturers have embraced rFC production, not all have been so supportive. The federally mandated monopoly for LAL production granted via licenses to produce LAL by harvesting animals from public waters gives LAL manufacturers little incentive to change.
(ii) The pace of change – Lonza submitted an rFC validation to USP Pharmaceopeial Forum some 10 years ago.7 Conversely, the US Food and Drug Administration (FDA) has proposed the use of rFC8 as well as recently accepting the use of rFC in lieu of LAL for two new injectable products.9,10 Pharmacopoeias are either currently adopting (Europe in 2.6.32) or have published the intention to include rFC at a future date (USP and Japan).
(iii) Quality control (QC) strategies – The strategy for assessing supply chain risks in QC testing of medications, vaccines and other medical supplies has remained static for several decades. However, for the reasons described here, a changing attitude in supply chain risk assessment may encourage pharmaceutical and medical device companies to reassess the supply and demand profile for critical QC test reagents necessary for the continued production of their medical products.
Risk of reliance on LAL for endotoxin testing – demand and supply perils
Risk assessment is a necessary part of supply chain management, especially for vital medical supplies.11
- Supply risk assessment is a determination of whether the supply of endotoxin tests can be maintained or may be drastically altered in terms of price or availability.
- Demand risk assessment is a determination of whether increases in the need for endotoxin testing worldwide will change and if it can potentially outpace supply.
Has the availability of the horseshoe crab for harvesting of its blood and subsequent production of LAL been taken for granted, thus overlooking the inherent risks for pharmaceutical drugs and medical device production? Risk assessment, in terms of supply chain assurance, should include a healthy dose of questioning and answering the simple queries focused on LAL: “What problems could occur? How can we optimally prevent these problems? How can we best resolve such problems if and when they occur?”.
The FDA/ICH Q9 Guidance12 document frames this in three questions:
- What might go wrong?
- What is the likelihood (probability) it will go wrong?
- What are the consequences (severity)?
The common practice of scoring risk (R = L X S) means that even if the likelihood of failure (or inability to release product) is low, if the severity of the event is high, then the risk remains high. Frank et al. list “market stock” as a “catastrophic” risk level for important products (outlined in Table 3 of their article)13 and LAL would certainly be listed as absolutely necessary for product release testing if only LAL-dependent endotoxin tests are used.
The current COVID-19 pandemic provides an unfortunate example of the fragility of our supply chains for vital medical products”
Given the premise that there is some possibility of combined supply/demand constraints related to LAL, what is the contingency plan of pharmaceutical and medical device companies? Several steps could be taken quickly, including reverting to the use of rabbit pyrogen testing, switching to more limited testing of various process constituents or use of alternate methods such as rFC-containing endotoxin assays. However, the use of rabbit pyrogen has been reduced for a host of reasons and reversion to limited testing would be viewed as unacceptable from a modern quality assurance perspective. Rising demand, foreseeable decreasing supply or the increased vigilance to detect possible risks require a recalculation of the existing risk appraisals for LAL and our dependence on it for assessment of endotoxin.14
In terms of risk assessment response, endotoxin assay users should be prepared to source alternative resources in the event of any of the aforementioned situations which lead to an unbalanced supply or demand. In the event of supply disruption, reagent manufacturers would be tasked with deciding who gets a limited supply and how much they should pay for it. Therefore, both potential supply and demand shocks should be conceivably encompassed in a risk assessment. The ramp up of endotoxin testing using rFC will require advance preparation for expanding cell culture production to meet the testing demand, equipment qualification, user training, validation and drafting of new procedures. Pharmaceutical and medical device industry diversification of endotoxin testing, completed as a risk mitigation strategy, could therefore help to avert a future industry-wide crisis.
Conclusion
When rabbit pyrogen testing was replaced by LAL‑containing endotoxin assays, few people argued that LAL was inadequate because it cannot detect any pyrogen but endotoxin. Rather, the medical industries accepted the enhanced specific detection of endotoxin alongside the potential increased risk of non-endotoxin pyrogens in order to decrease the dominant risk of their products (increasing process coverage, endotoxin sensitivity, etc.) for the ultimate benefit of patients.
The use of both LAL and rFC testing will likely continue for many years, with either being an acceptable test. However, the sole reliance on LAL‑dependent assays seems to be an unacceptable risk control strategy, especially when considering the continuous supply of safe medical products to meet market demand and assure ongoing patient safety. In addition to “risk mitigation”, it is appropriate to view endotoxin testing that is not reliant on LAL as a modernisation of the LAL assay via the use of a more highly characterised and standardised material.
Disclaimer
The views and opinions expressed are those of the authors and do not necessarily represent the views, positions or opinions of bioMérieux Inc., bioMérieux SA or any of its holdings or subsidiaries.
About the authors




References
- The Delaware Bay’s Horseshoe Crab population has declined by 90% over the last 15 years mostly due to overharvesting and habitat degradation. As the number of Horseshoe Crabs have decreased, so have the number of eggs available for consumption by migrating shorebirds. Shorebird population numbers are therefore plummeting as well, as many cannot gain the amount of energy needed to complete their migrations. Wetlands Institute, accessed June 10, 2020.
- Results indicated that significant declines occurred in at least one dataset in all areas except the Southeast and Florida… Conservation status of the American horseshoe crab, (Limulus polyphemus): a regional assessment, Smith et al., Rev Fish Biol Fisheries (2017) 27:135–175.
- https://www.iucnredlist.org/species/11987/80159830
- https://www.iucnredlist.org/species/21309/133299524
- USP Global Public Policy Position Key Elements to Building a More Resilient Supply Chain, accessed online at USP.org 05/09/2020 https://www.usp. org/sites/default/files/usp/document/our-impact/ covid-19/global-policy-supply-chain.pdf
- If oil spilled off SC’s coast, a huge current would make it ‘impossible to control’, Tony Bartelm, The Post and Courier, Sept. 2018. https://www.postandcourier. com/news/special_reports/if-oil-spilled-off-scs-coasta- huge-current-would-make-it-impossible-to-control/ article_9e1e534c-9fce-11e8-887d-63b1602d3df9.html, accessed June 6, 2020.
- Loverock et al., A recombinant Factor C procedure for the detection of Gram-negative bacterial endotoxin, United States Pharm. Convention, Inc., 36(1): 321-329.
- FDA Q&A Guideline, 2012.
- Lilly’s new migrane drug: Emgality® (2019)
- https://www.prnewswire.com/news-releases/chmprecommends- approval-of-lillys-new-fast-actingmealtime- insulin-to-improve-glycemic-control-inadults- with-diabetes-300996785.html
- On the Value of Mitigation and Contingency Strategies for Managing Supply Chain Disruption Risks, Brian Tomlin, Management Science, May 2006.
- https://www.fda.gov/media/71543/download
- Quality Risk-Management Principles and PQRI Case Studies, A PQRI expert working group provides case study examples of risk-management applications, Jul 02, 2011, Pharmaceutical Technology, Volume 35, Issue 7.
- Changing Global Perspectives on Horseshoe Crab Biology, Conservation and Management, Carmichael et al., Springer, 2015
Issue
Related topics
Drug Safety, Drug Supply Chain, Endotoxin, Endotoxin Detection, Manufacturing, Supply Chain, Sustainability









