Optimising spray-dried solid dispersions to improve solubility
Posted: 8 April 2021 | Javier Gurrea (Idifarma) | No comments yet
Manufacturing drug products with low solubility APIs (BCS type II and IV) has its challenges, in this article, Javier Gurrea, a Spray Drying Manufacturing Scientist at Idifarma, explains why spray drying offers a great way to overcome them.


Today’s active pharmaceutical ingredients (API) are increasingly insoluble and that can pose serious problems for formulators looking to manage bioavailability and dosing of their formulations. Moreover, the trend is building: around 40 percent of new potentially active chemical compounds are poorly soluble in water or lipophilic solutions.1
These drugs are classified in the Biopharmaceutical Classification System (BCS) as Class II (low solubility and high permeability) or Class IV (low solubility and low permeability).2
Considering that the gastrointestinal bioavailability of a chemical compound is determined by its solubility and permeability, a low water-solubility value can result in poor bioavailability. Dealing with a compound’s specific bioavailability issues can be challenging and not all of them can be overcome without some kind of intervention in formulation and manufacture.
Improving the oral bioavailability of low solubility actives is one of the greatest challenges for new product development. As such, a number of formulation and manufacturing techniques are available to support the formulation and development of insoluble BCS type II and IV actives.
One of these is spray drying, which can be a highly valuable tool in reformulating existing drug products. It is a perfect technique for exploring new development routes along the 505(b)(2) regulatory pathway and creating better-performing, more marketable products (eg, soft-gel capsules into tablets or injectable therapies into oral products).
Common solubility-boosting strategies include physical-chemical modifications, which include the use of surfactants and co-solvents, etc; pH modulation, which involves complex and salt formation; and physical modification, such as particle size reduction, changes in the crystalline structure and the use of amorphous forms to administer the dosing of the drug.3
Solid dispersions: ready to rescue programme potential


In the context of contemporary drug commercialisation strategy solid dispersions (SDs) are increasingly being recognised as one of the more reliable efficient strategies for optimising the solubility and dissolution characteristics of low aqueous solubility characteristics.
Dhirendra et al., adopted the definition given by Chiou and Riegelman and defined the term solid dispersion as a group of solid products consisting of at least two different components, generally a hydrophilic matrix and a hydrophobic drug. The matrix can be either crystalline or amorphous. The drug can be dispersed molecularly, in amorphous particles (clusters) or in crystalline particles.4,5
There are several fundamental characteristics that make amorphous SD (ASD) particles more soluble than the crystalline particles and provide much of the rationale behind why this technology is increasingly being chosen to address the toughest drug solubility issues.
Spray drying, the solid dispersion technique for today’s formulations
As a technique for processing SDs, spray drying is accessible, repeatable, uncomplicated and offers developers a cost-effective effective way to improve the solubility and bioavailability of their formulations.
Looking across all the ASD manufacturing techniques, spray drying stands out for several reasons, because:
- It offers a continuous process while feedstock solution is present
- It creates a homogenous product while controlling for particle size and morphology
- The evaporation process is instantaneous making it suitable for heat-sensitive products; and
- Ultimately, it is a highly automatable, scalable process.6
Spray drying is a mature technology. It is also broadly applied across commercial industry including the pharma, food and dairy processing and chemical sectors. With all these possible applications, the market for spray drying technology is projected to increase substantially. According to one market analysis group, in 2020 the market was valued at approximately $4.5 billion but was projected to reach $6.96 billion by 2025, achieving a compound annual grow rate of 6.5 percent.7
Sprayed dispersions build versatile soluble particles efficiently
Spray drying builds solubility into actives in a variety of ways. One top attribute is the process can reduce particle size, compared to the original particle size of the starting product. Spray drying increases the surface area of the particles and therefore, improves dissolution rates.8 Spray drying ASDs also offers drug developers manufactured particles with several useful attributes to help deliver insoluble APIs:
- Improved wettability. Solid dispersions improve the wettability of the particles, even in the case of carriers without surface activity because of the hydrophilic properties of polymers. On the other hand, carriers with surface-active properties can considerably increase the wettability of the drug and, therefore, influence the dissolution profile of the drug through co-solving effects.9,10
- Porous particle morphologies. Spray-dried ASDs offer another important feature – they can be manufactured to provide a high degree of porosity. This characteristic can be desirable but achieving it effectively depends on precisely controlling formulation factors, such as the type of carrier and on careful management of the manufacturing process.11
- Rapid solvent removal. A key aspect of this process is the rapid evaporation of the solvent, which makes it capable of being applied to obtain amorphous solid dispersions. Additionally, the rate of this evaporation process allows the use of this technology in thermolabile products.
- Amorphous form. Finally, the most relevant aspect of spray drying formulations is that APIs in an amorphous state improve solubility because they do not need extra energy to break the crystalline structure during the solubilisation process.12
Optimising spray drying for better bioavailability
The conventional spray-drying equipment consists of a drying chamber where a liquid stream (solution or suspension) is atomised through a nozzle, forming fine droplets that come into contact with a hot gas which allows the solvent to evaporate resulting in the formation of dry particles.
In the first place, dry particles are separated from the gas stream by a cyclone separator and then the finest or smaller particles are retained by a filtering system, commonly bag filters. The scheme of a typical closed cycle spray-drying equipment is shown in Figure 1.
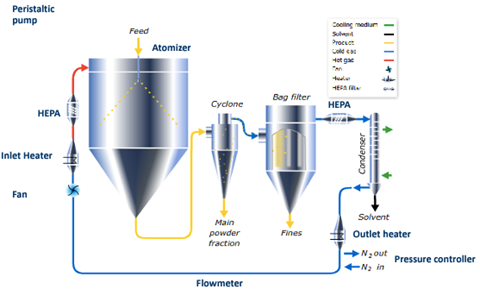

Figure 1: Schematic representation of a conventional closed cycle spray-drying equipment (structure based on the GEA Niro Mobile Minor® model).13
The spray-drying process consists of four basic, but highly optimisable steps for best effect:
1. Atomisation
Depending on the mechanism and energy used to achieve the atomisation of the liquid stream, different types of atomisers are available. As such, depending on the atomisation device chosen, as well as the properties of the feedstock solution (and the established process parameters), a certain droplet size and, consequently, a specific particle size distribution can be obtained. The key here is to choose the atomiser device best suited to the features of the equipment, including the feeding liquid characteristics and target particle size distribution.
2. Mixing the liquid with the drying gas
Once the liquid stream has been atomised, the droplets come into contact with the drying gas. This process takes place in the drying chamber where the gas is introduced by a dispenser and distributed equally throughout the chamber.
Depending on the flow pattern of the liquid and the gas stream, the following types of gas-liquid contact can be distinguished: co-current, counter-current or a mixed flow system. Co-current systems are the most widely used because they can be applied to thermolabile substances because, unlike a counter-current system, the dried particles are exposed to the lower temperature zone of the drying chamber.14
3. Evaporation of the solvent
When the gas stream comes into contact with the droplets, the solvent evaporates, yielding dry particles. For best results, and to achieve desired characteristics and quality reliably, it is critical to control key equipment variables during this stage:
- The volume of the drying chamber must be adequate to optimise the time in which the gas and the atomised solution are in contact
- The particles must be sufficiently dry before contacting the surface of the equipment
- The height and diameter requirements of the chamber will depend on the type of atomiser used
- Depending on the nature of the solvent to be evaporated, one gas or another can be used (inert gases are used for organic solvents to minimize the risk of explosion).
4. Separation of the dry particles from the gas stream
The most commonly used separation device to separate dry particles is a high-performance cyclone in which the particles are able to escape from the generated vortex, thus being separated from the gas stream. There is also the possibility of performing the separation in the drying chamber itself (common in laboratory-scale equipment). On the other hand, the finest particles obtained during the process can be collected using a filter system.
Conclusions
Spray drying ASDs for better dissolution is increasingly recognised by pharma’s top manufacturers as one of the most efficient and economical ways to overcome bioavailability problems with BCS type II and IV drugs by obtaining thermally stable solid dispersions. Although the process is well understood, it is highly technical and requires technical capability and operational excellence to accomplish successfully. Therefore, not every drug manufacturer possesses the specialised capabilities required to get the best results from spray dried dispersions.
About the author
Javier Gurrea is a Pharmaceutical Development and Spray Drying Manufacturing Scientist at Idifarma. He has been involved in numerous projects based especially on amorphous solid dispersions, as well as working on other applications of this technology including micro and nanoparticles. Javier has a double degree in Pharmacy and Nutrition from the University of Navarra in Spain.
Idifarma is a specialist CDMO offering spray drying manufacturing services for clinical and commercial purposes for niche and highly potent drugs since 2001.
References
- Lipinski C. Avoiding investment in doomed drugs. Curr Drug Discov. 2001 Jan 1;17–9.
- Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System Guidance for Industry. US Food and Drug Administration. 2017.
- Savjani KT, Gajjar AK, Savjani JK. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012;2012.
- Dhirendra K, Lewis S, Udupa N, Atin K. Solid dispersions: a review. Pak J Pharm Sci. 2009 Apr;22(2):234–46.
- Chiou WL, Riegelmant S. Pharmaceutical sciences Pharmaceutical Applications of Solid. J Pharm Sci. 1971;60(9):1281–302.
- Mujumdar AS. Handbook of industrial drying. CRC/Taylor & Francis. 2007.
- Spray Drying Equipment Market – Global Industry Analysis, Market Size, Opportunities and Forecast, 2019 – 2026 [Internet]. Acumen Research and Consulting. [accessed 2021 Mar 3]. Available from: https://www.acumenresearchandconsulting.com/spray-drying-equipment-market
- Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000 Jul;50(1):47–60.
- Sekiguchi K, Obi N, Ueda Y. Studies on absorption of eutectic mixture. Ii. Absorption of fused conglomerates of chloramphenicol and urea in rabbits. Chem Pharm Bull (Tokyo). 1964 Feb;12:134–44.
- Van den Mooter G, Weuts I, De Ridder T, Blaton N. Evaluation of Inutec SP1 as a new carrier in the formulation of solid dispersions for poorly soluble drugs. Int J Pharm. 2006 Jun 19;316(1–2):1–6.
- Ghaderi R, Artursson P, Carlfors J. Preparation of biodegradable microparticles using solution-enhanced dispersion by supercritical fluids (SEDS). Pharm Res. 1999 May;16(5):676–81.
- Pokharkar VB, Mandpe LP, Padamwar MN, Ambike AA, Mahadik KR, Paradkar A. Development, characterization and stabilization of amorphous form of a low Tg drug. Powder Technol. 2006 Sep;167(1):20–5.
- Types of spray drying installations [Internet]. GEA Niro. 2019 [cited 2019 May 6]. Available from: https://www.gea.com/es/expert-knowledge/milk-powder-manufacture/…
- Cal K, Sollohub K. Spray drying technique. I: Hardware and process parameters. J Pharm Sci. 2010 Feb;99(2):575–86.









