Balancing regulations for weighing in a GMP quality control laboratory
Posted: 23 April 2021 | Dr Bob McDowall (R D McDowall Limited) | No comments yet
The importance of analytical balances in laboratory operations demands that they be fit for purpose over the operating range and acceptance criteria specified in the laboratory user requirements specification. In this article, Bob McDowall, Director of R D McDowall Limited, discusses the regulatory requirements for analytical balances operating in GMP and GLP laboratories and discusses how to solve some of the data integrity issues that can arise from their use.


Introduction
Analytical balances are critical instruments in any laboratory as they impact the outcomes of analyses either directly or indirectly. The direct impact is through accurate weighing of analytical reference standards and samples and the indirect impact is via the accurate preparation of buffers, solutions and chromatography mobile phases.
When operated in an unregulated laboratory, an analytical balance should follow sound analytical science principles, such as correct siting of the instrument with calibration checks and preventative maintenance. However, what is the situation in a Good Laboratory Practice (GLP) or Good Manufacturing Practice (GMP) regulated laboratory? In this article we will consider the regulatory requirements for an analytical balance operating in a GMP laboratory such as analytical development or quality control. The requirements are similar for a GLP laboratory, apart from the requirement to follow applicable Pharmacopoeial general chapters. We will also discuss some of the data integrity issues involving analytical balances.
Regulations and guidance for analytical balances
The regulations and guidance that we will discuss are shown in Figure 1. These will be discussed as we progress through the life cycle of an analytical balance. We will also discuss some of the requirements that impact balances in the various data integrity guidance documents that have been published since 2015.
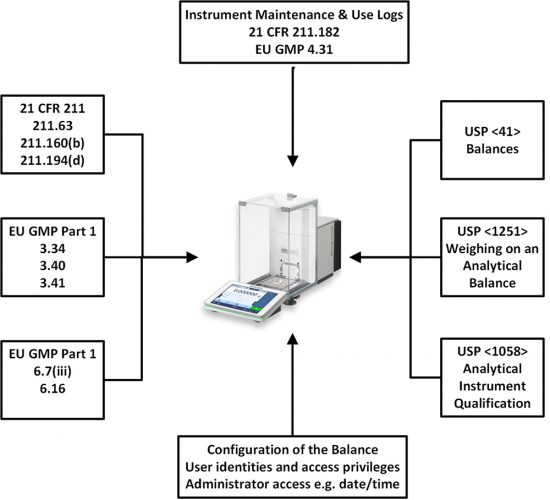

Figure 1: GMP Regulations and United States Pharmacopoeia (USP) General Chapters Impacting
Analytical Balances.
Purchase and qualification of a balance
The US and EU GMP regulations for equipment only require a balance to be of appropriate design, adequate size and be suitably located for intended use.1,2 It is left to an individual organisation to interpret these requirements. Help is at hand from a general chapter in the United States Pharmacopoeia (USP) in <1058> on Analytical Instrument Qualification (AIQ).3 First, this requires a laboratory to specify the operating range of the balance and the repeatability and accuracy of measurement (USP minimum weight) as mandated by USP <41>4 in a user requirements specification (URS) that defines the balance’s intended use. The URS is used to select the instrument and supplier based on this (design qualification [DQ]). This is the worst part of the instrument life cycle, as most laboratories do not write down their requirements or simply copy a supplier’s specification. The latter is not advised as a supplier specification may involve a wider range of operation than a laboratory requires but still must be qualified and calibrated.
Once purchased, the installation qualification (IQ) demonstrates that the instrument has been sited correctly taking into account environmental conditions, eg, no drafts or vibrations impacting the balance operation and checks that it works acceptably. The balance is then tested using a range of calibrated masses against the URS operating range and accuracy during the operational qualification (OQ). This is a similar approach as outlined in Annex 15 on Qualification and Validation.5 If the balance performs a weight uniformity calculation this must be verified as required by 21 CFR 211.68(b)1 and EU GMP 6.16.2
To print or not to print?
A key requirement is if a printer should be included in the laboratory URS. The answer is an unequivocal yes. Any balance needs a printer to record the work performed. Recording any weight by observation is unacceptable, especially in a regulated laboratory, due to potential for error or data falsification. The 2015 MHRA data integrity guidance explicitly stated that balances must have a printer.6 As an auditor, an analytical balance without a printer and recording weights by observation would be a critical observation.
A much better approach than using a printer is to interface a balance to an informatics application, such as an instrument data system, laboratory information management system (LIMS) or electronic laboratory notebook (ELN), to transfer the weighing sequence to a database on a secure server. This would eliminate paper recording followed by manual entry into another application with transcription error checks, replacing it with an electronic workflow that ensures GMP compliance and data integrity via technical controls in the workflow.7
Access privileges and instrument log book
Before the balance is placed into operational use there are two tasks to complete:
- Set up the balance for named users and their access privileges. It is important that the balance is configured to ensure that there are unique user identities for each user to ensure attribution to an individual. It is important that nobody, except the administrator who has no conflicts of interest, can change the date and time stamp on the balance.
- Establish an instrument maintenance and use log as required by 21 CFR 211.1821 and EU GMP 4.31.8 All activities must be recorded in chronological order. A common mistake is to separate maintenance activities from use of the instrument. Reading the regulations makes it clear that ALL activities must be in chronological order. There also needs to be space for the second person reviewer to document they have reviewed the weighing tasks for the work they are checking. Second, the instrument owner needs to check the overall performance of the instrument on, say, a monthly basis so that the balance is continuing to operate as expected against the URS requirements and acceptance criteria.
Analyst training
In any regulated laboratory there must be a procedure with training for using the analytical balance. To help in this regard, the USP have published a good practice guide in USP <1251> called weighing on an analytical balance.9 Portions of this general chapter can be used to structure the standard operating procedure (SOP) and the associated training for staff.
Performance qualification and point of use checks
All balances have a life cycle that follows the 4Qs model presented in USP <1058> that includes preventative maintenance, repair and requalification”
Owing to the criticality of analytical balances, most laboratories perform a daily point of use check to show that the balance is still functioning correctly over the operating range. This is undertaken using a calibrated mass set and is a portion of USP <1058> Performance Qualification (PQ).3 In the case of an analytical balance, the checks must related back to the requirements and accuracy requirements in the laboratory URS. The internal calibration of the balance can be used instead, but the operation must be checked using external calibrated masses at longer intervals. However, if an external mass check is out of acceptance limits ALL weighing since the last correct external mass check are suspect and must be investigated, for more information please see question 1 in the FDA Questions and Answers on Current Good Manufacturing Practices –Laboratory Controls: Level 2 Guidance.10
One of the roles of the instrument owner must be to confirm that over time the balance performance is consistent and that there are no adverse trends. If a PQ check is out of tolerance, the balance must be taken out of use as required by 21 CFR 211.160(b)(4)1 and the issue resolved by a service engineer. This is another part of PQ: preventative service, maintenance and repair.3 All activities must be documented both in the instrument log and cross referenced with any documentation that the engineer leaves.
Requalification of the balance is usually performed after the annual preventative maintenance visit and after repair to demonstrate that the instrument can still meet the requirements of accuracy and precision stated in the laboratory URS.
Conclusions
The criticality of an analytical balance in laboratory operations demands that the balance be fit for its intended use over the operating range and acceptance criteria specified in the laboratory URS. It is essential that there is input from GMP regulations and USP <41>. All balances have a life cycle that follows the 4Qs model presented in USP <1058> that includes preventative maintenance, repair and requalification. Any balance must have a printer as a minimum and be configured for all users to be uniquely identified. Procedures and training must ensure that use of a balance is correct and recorded chronologically in the instrument log book.
About the author
Dr Bob McDowall is an analytical chemist with nearly 50 years’ experience including 15 years working in the pharmaceutical industry. Bob has been a consultant for nearly 30 years and has been involved with computer validation for 35 years. He is a contributor to the GAMP Good Practice Guide for Validation of Laboratory Computerised Systems 2nd edition and a contributor and reviewer of the GAMP Guide on Records and Data Integrity and two associated Data Integrity Good Practice Guides. He is the author of Data Integrity and Data Governance: Practical Implementation for Regulated Laboratories and Validation of Chromatography Data Systems 2nd edition.
References
- CFR – Code of Federal Regulations Title 21. US Food and Drug Administration (FDA). 2008.
- EudraLex – Volume 4 Good Manufacturing Practice (GMP) Guidelines, Chapter 6 Quality Control. European Commission. 2014.
- USP General Chapter <1058> Analytical Instrument Qualification. United States Pharmacopoeia Convention. 2017.
- USP General Chapter <41> Weights and Balances. US Pharmacopoeia. 2014.
- EudraLex – Volume 4 Good Manufacturing Practice (GMP) Guidelines, Annex 15 Qualification and Validation. European Commission. 2015.
- MHRA GMP Data Integrity Definitions and Guidance for Industry 2nd Edition. Medicines and Healthcare products Regulatory Agency. 2015.
- McDowall RD. How Can LIMS Help Ensure Data Integrity? LCGC Europe. 2016. 29(6): p. 310-316.
- EudraLex – Volume 4 Good Manufacturing Practice (GMP) Guidelines, Chapter 4 Documentation. European Commission. 2011.
- USP General Chapter <1251> Weighing on an Analytical Balance. US Pharmacopoeia. 2017.
- FDA Questions and Answers on Current Good Manufacturing Practices—Laboratory Controls: Level 2 Guidance [Internet]. US Food and Drug Administration. Available from: https://www.fda.gov/drugs/guidances-drugs/questions-and-answers…









